Tricia Suvari, Esq. | | (1) | Mr. Jeffrey Farrow joined the Company as Chief Financial Officer on April 4, 2016. |
(2)59 | Ms.Tricia Suvari joined the Company as | | Chief Legal Officer on October 11, 2016. |
(3) | Dr. Hing Sham was appointed as Senior Vice President, Research effective as of May 1, 2016. |
(4) | Mr. Radovich was appointed as Senior Vice President, Operations effective as of September 1, 2016. |
Executive Officers The biographies of our executive officers, other than Dr. Love, whose biography is set forth above, appear below. JeffreyFarrow has served as our Chief Financial Officer since April 2016. Mr. Farrow previously served as chief financial officer of ZS Pharma, Inc., a biopharmaceutical company, which was acquired by AstraZeneca in December 2015. Prior to ZS Pharma, he served as the chief financial officer at Hyperion Therapeutics, Inc., a commercial pharmaceutical company, from July 2010 until May 2015 where he was part of the team responsible for the successful regulatory approval and commercial launch of RAVICTI® for the treatment of urea cycle disorders. He previously served as vice president of finance at Evotec AG, a drug discovery and development company. Prior to Evotec, Mr. Farrow served as vice president of finance and chief accounting officer at Renovis, Inc., a drug discovery and development company, which was acquired by Evotec AG. Earlier in his career, Mr. Farrow spent seven years working in the audit practice of KPMG LLP. Mr. Farrow holds a B.A. in business administration with a concentration in corporate finance from California State University at Fullerton and is a certified public accountant (inactive). TriciaSuvari,Esq,Brian Cathers, Ph.D. has served as our Chief LegalScientific Officer since October 2016. From May 2000 until June 2009, Ms. SuvariFebruary 2019. Dr. Cathers previously served in several senior rolesas executive director and head of drug discovery at CV Therapeutics, Inc., a biopharmaceutical company (acquired by Gilead Sciences, Inc. in 2009), ultimatelyCelgene Corporation, or Celgene, from November 2015 to February 2019, and as senior vice president, general counseldirector of biochemistry and chief compliance officer of CV Therapeutics.structural biology at Celgene from October 2013 to November 2015. At Celegene, Dr. Cathers’ teams produced eight new development candidates and advanced five investigational drugs into clinical testing. Prior to CV Therapeutics, from May 1991 until May 2000, shejoining Celgene, Dr. Cathers served as corporate counselsenior group leader and director at Genentech,NewBiotics, Inc., in increasingly senior roles. From February 2012 until July 2016, Ms. Suvari served as a vice president and general counsel at thenon-profit Peninsula Open Space Trust, and from early 2011August 2000 to February 2012 she2004, where he oversaw enzymology and biophysical chemistry research, and was part of small team that developed a colorectal cancer drug from basic research to clinical testing. Prior to joining NewBiotics, he served as an independent consultant supporting biopharmaceutical companies. Ms. Suvari earned her Bachelor of Sciences degreeenzymologist at Axys Pharmaceuticals. Dr. Cathers holds a B.S. in Geology and Geophysicschemistry from YaleEmporia State University and her J.D. degreean M.S. and Ph.D. in medicinal chemistry from Harvard Law School.the University of Kansas.
JungE.Choi has served as our Chief Business and Strategy Officer since April 2015. From April 2014 to March 2015, Ms. Choi served as senior vice president, corporate development for InterMune, Inc., a biotechnology company (acquired by Roche Holding AG in 2014), and served as an adviser on strategy and business development to InterMune from March 2013 to April 2014. Prior to joining InterMune, from February 2011 to March 2013, Ms. Choi led corporate and business development for Chimerix, Inc., a biopharmaceutical company, as a consultant and senior vice president, corporate development. Prior to that, from August 2001 to August 2010, Ms. Choi held various management positions at Gilead Sciences, Inc., a biopharmaceutical company, including leadership of business development, licensing, and mergers and acquisition activities. During her tenure at Gilead Sciences, Ms. Choi built and oversaw the corporate development group, and led the U.S. commercial launch of Hepsera® for the treatment of the hepatitis B virus. Ms. Choi holds a B.A. in human biology and an M.B.A. from Stanford University. HingEric Fink was appointed our Chief Human Resources Officer in August 2019. Prior to joining us, from 2010 until July 2019, he most recently served as Vice President, Human Resources, at Jazz Pharmaceuticals, a global biopharmaceutical company. While there, he held a variety of human resource senior leadership positions to develop human capital and organizational development strategies and scale the global HR operating model to better serve the expanding business. From 2008 to 2009, he held a leadership position in the sales training organization at Bayer Healthcare, a multinational pharmaceutical and life sciences company. From 1999 to 2008, he held various roles at GlaxoSmithKline, a global healthcare company, across a wide spectrum of the U.S. commercial function, including Sales, Sales Training, Commercial Analytics, and Sales Management. He received a B.S. in Biology from Pennsylvania State University and an M.S. in Organizational Leadership from Mercyhurst University.
Sham,Ph.D.David L. Johnson has served as our Chief Commercial Officer since March 2018. From October 2003 until February 2018, Mr. Johnson served in roles of increasing responsibility in the commercial organization at Gilead Sciences, Inc., a biopharmaceutical company, ultimately as vice president, sales and marketing, for Gilead’s Liver Disease Business Unit. At Gilead, Mr. Johnson was responsible for the commercial launch of Gilead’s hepatitis C treatments Sovaldi®, Harvoni®, Epclusa® and Vosevi®, hepatitis B treatment Vemlidy®, and HIV treatments Complera® and Stribild®. Prior to Gilead, from April 1992 to September 2003, Mr. Johnson served in various roles in sales, product marketing, business development, global commercial strategy and portfolio development at GlaxoSmithKline PLC, a British pharmaceutical company. Mr. Johnson holds a B.A. in business marketing from the University of Puget Sound and an M.B.A. from the Kenan-Flagler Business School at the University of North Carolina.
Tricia Suvari, Esq., has served as our Chief Legal Officer since October 2016. From 2000 until 2009, Ms. Suvari served in several senior roles at CV Therapeutics, Inc., a biopharmaceutical company (acquired by Gilead Sciences, Inc. in 2009), ultimately as senior vice president, general counsel and chief compliance officer. Prior to CV Therapeutics, from 1991 until 2000, she served as corporate counsel at Genentech, Inc., in increasingly senior roles. From February 2012 until July 2016, Ms. Suvari served as a vice president and general counsel at thenon-profit Peninsula Open Space Trust, and from early 2011 to February 2012 she served as an independent consultant to biopharmaceutical companies. Ms. Suvari earned her Bachelor of Sciences degree in Geology and Geophysics from Yale University and her J.D. degree from Harvard Law School. EXECUTIVE COMPENSATION Compensation Discussion and Analysis This Compensation Discussion and Analysis, or CD&A, describes our executive compensation program and the 2019 compensation for: (i) each individual who served as our principal executive officer during 2019; (ii) each individual who served as our principal financial officer during 2019 and (iii) our three most highly compensated executive officers during 2019 other than the individuals set forth above in clauses (i) and (ii), all of whom we refer to collectively as our named executive officers, or NEOs. This CD&A should be read with the compensation tables and related disclosures for our NEOs. Our NEOs for 2019 were as follows: Ted W. Love, our President and Chief Executive Officer, or CEO; Jeffrey Farrow, our Chief Financial Officer; Brian Cathers, our Chief Scientific Officer; David L. Johnson, our Chief Commercial Officer; and Joshua Lehrer-Graiwer, our former Chief Medical Officer. Management Changes in 2019 and 2020 We hired Dr. Cathers in February 2019. On September 17, 2019, our Board of Directors appointed Dr. Lehrer-Graiwer as our Chief Medical Officer, effective October 1, 2019. Prior to his appointment as our Chief Medical Officer, Dr. Lehrer Graiwer served as our Senior Vice President, Research, since May 2016. He was Senior Vice President, Chemistry,Clinical Development. In February 2020, Dr. Lehrer-Graiwer resigned from July 2014 tohis employment with our company, effective April 2016. Most recently, from January 2013 to July 2014, Dr. Sham served as head of research and development at iOneWorldHealth/Path.org (PATH), anon-profit pharmaceutical development organization. Prior to that, from September 2006 to November 2012, he served as senior vice president of research and head of chemical sciences at Elan Pharmaceuticals, Inc.,17, 2020. Overview We are a biopharmaceutical company where he led the chemistry team indedicated to the discovery, development and delivery of two clinical candidateslife-changing treatments that provide hope to underserved patient communities. Founded in 2011, we are delivering on our goal to transform the treatment and care of sickle cell disease, or SCD, a lifelong, devastating inherited blood disorder that is marked by red blood cell destruction and occluded blood flow and hypoxia, leading to anemia, stroke, multi-organ failure, severe pain crises, and shortened patient life span. Notably, 2019 was a pivotal year in our corporate history as we became a commercial-stage company with a marketed drug. In late November 2019, we received U.S. Food and Drug Administration, or FDA, accelerated approval for our first product, Oxbryta® (voxelotor) tablets for the treatment of Alzheimer’s disease. From July 1983SCD in adults and children 12 years of age and older. Oxbryta, an oral therapy taken once daily, is the firstFDA-approved treatment that directly inhibits sickle hemoglobin polymerization, an underlying cause of SCD. This FDA approval was three months ahead of the FDA’s Prescription Drug User Fee Act, or PDUFA, target action date of February 26, 2020, and we began to August 2006, Dr. Sham worked at Abbott Laboratories Inc., a global healthcare company, where hemake Oxbryta available to patients through our specialty pharmacy partner network in early December 2019. We are conducting and his team discoveredplan to conduct additional studies of Oxbryta, including our Phase 2a HOPE-KIDS 1 Study (an open-label, single- and advanced 10 clinical candidates spanning cardiovascular disease, HIV, oncologymultiple-dose Phase 2a study that is evaluating the safety, tolerability, pharmacokinetics and diabetes. His24-year tenure at Abbott Laboratories culminatedexploratory treatment effect of Oxbryta in his appointmentpediatric patients aged four to 17 years with SCD) and, as a distinguishedcondition of accelerated approval, our Phase 3 HOPE-KIDS 2 Study (a post-approval confirmatory study we initiated in December 2019 that is using transcranial Doppler, or TCD, flow velocity to seek to demonstrate a decrease in stroke risk in children two to 15 years of age). We also expect to conduct additional clinical studies of Oxbryta, including to seek to expand the potential approved product label into younger pediatric populations. Beyond Oxbryta, we are also engaged in other research fellowand development activities. For example, we are advancing our SCD pipeline with inclacumab, ap-selectin inhibitor in global pharmaceutical discovery. Dr. Shamdevelopment to address pain crises associated with the disease. In addition, our drug discovery team is working on new targets to develop the next generation of treatments for SCD. As part of those efforts, we regularly evaluate opportunities toco-inventorin-license, of Norvir®acquire or invest in new business, technology or assets or engage in related discussions with other business entities. In December 2019, we entered into a license and collaboration agreement, or the primary inventor of Kaletra®, Abbott Laboratories’ first and second-generation HIV protease inhibitors approved for the treatment of HIV. Dr. Sham has published more than 180 scientific articles in peer-reviewed journals and is a named inventor on 81 issued U.S. patents. Dr. Sham was named Hero of Chemistry by the American Chemical Society in 2003. Dr. Sham holds a Ph.D. in synthetic organic chemistry from the University of Hawaii and completed his post-doctoral training in the department of chemistry at Indiana University. PeterRadovich has served as our Senior Vice President, Operations, since September 2016, and was our Vice President, Program Leadership and Business Strategy from November 2014 to August 2016. Prior to that, in September 2006, he joined OnyxSyros Collaboration, with Syros Pharmaceuticals, Inc., a biopharmaceutical company (acquired by Amgen, Inc.),or Syros, to discover, develop and served as vice president,commercialize novel therapies for SCD and beta thalassemia.
Corporate Performance Highlights Our executive compensation program leadership from February 2014seeks to November 2014incentivize and as senior director from August 2011 to February 2014, during which time he led the company’s global, cross-functional product team responsible for the development and commercializationreward strong corporate performance. Highlights of Kyprolis®. Prior to joining Onyx, from 2004 to 2006, Mr. Radovich was at Chiron Corporation, a biopharmaceutical company (now Novartis AG) in product marketing supporting Proleukin®(interleukin-2) in multiple oncology indications. Mr. Radovich holds a B.A. in biology and chemistry from Texas Christian University and an M.B.A. from Washington University in St. Louis. COMPENSATION OF EXECUTIVE OFFICERS
Summary Compensation Table
The following table presents information regarding the total compensation earned by each individual who served as our chief executive officer at any time during the fiscal year ended December 31, 2016, our two other most highly compensated executive officers who were serving as executive officers as of December 31, 2016 and one individual who would have been included as one of the most highly compensated executive officers except that she was no longer serving as an executive officer at the end of 2016. We refer to these officers in this Proxy Statement as our named executive officers. The following table also sets2019 corporate performance are set forth information regarding total compensation awarded to, earned by and paid to each of these named executive officers during the fiscal year ending December 31, 2015, to the extent they were named executive officers in 2015.below.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | Name and Principal Position | | Year | | | Salary
($) | | | Stock Awards
($) | | | Option
Awards
($)(1) | | | Non-Equity
Incentive Plan
Compensation
($)(2) | | | All Other
Compensation
($)(3) | | | Total
($) | | | | | | | | | | Ted W. Love, M.D. | | | 2016 | | | | 479,375 | | | | 507,838 | (4) | | | — | | | | 230,000 | | | | 2,500 | | | | 1,219,713 | | | | | | | | | | President, Chief Executive Officer and Director | | | 2015 | | | | 437,500 | | | | 1,022,424 | | | | — | | | | 76,500 | | | | 750 | | | | 1,537,174 | | | | | | | | | | Jeffrey Farrow(5) | | | 2016 | | | | 298,718 | | | | — | | | | 1,613,816 | | | | 115,000 | | | | 2,500 | | | | 2,030,034 | | | | | | | | | | Chief Financial Officer | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Tricia Suvari(6) | | | 2016 | | | | 81,657 | | | | — | | | | 1,156,660 | | | | — | | | | 500 | | | | 1,238,817 | | | | | | | | | | Chief Legal Officer | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Eleanor L. Ramos, M.D.(7) | | | 2016 | | | | 350,949 | | | | — | | | | 1,113,389 | (8) | | | — | | | | 199,024 | (9) | | | 1,663,362 | | | | | | | | | | Former Chief Medical Officer | | | 2015 | | | | 358,333 | | | | — | | | | 175,112 | | | | 47,500 | | | | 750 | | | | 581,695 | |
(1) | • | | Regulatory and Commercial |
| ✓ | | In accordanceJune 2019, we announced final agreement with SEC rules, these columns reflect the aggregate grantFDA on the design of the post-approval confirmatory study of Oxbryta, our Phase 3 HOPE-KIDS 2 Study. |
| ✓ | | In September 2019, we announced that the FDA accepted for filing our New Drug Application, or NDA, seeking accelerated approval for Oxbryta and that the FDA granted Priority Review for the NDA, thus providing for asix-month review period and a PDUFA target action date fair value of restricted stockFebruary 26, 2020. |
| ✓ | | In the third quarter of 2019, we hired approximately 65 Sickle Cell Therapeutic Specialists, bringing our field team to approximately 75 members, on an accelerated hiring plan that met a product approval in November 2019, well in advance of the FDA’s PDUFA target action date. |
| ✓ | | In late November 2019, three months ahead of the FDA’s PDUFA target action date, the FDA granted accelerated approval for Oxbryta for the treatment of SCD in adults and option awards granted during 2016children 12 years of age and 2015older. |
| ✓ | | Upon the approval of Oxbryta, we launched GBT Source SolutionsTM, a comprehensive program for patients who are prescribed Oxbryta, their families and healthcare providers, which provides a wide range of practical, educational and financial support customized to each patient’s needs. |
| ✓ | | In early December 2019, Oxbryta was first made available in accordancethe United States through our specialty pharmacy partner network. |
| ✓ | | We completed the following financing transactions, ending 2019 with FASB ASC Topic 718. Pursuanta cash reserve that exceeded our 2019year-end cash goal: |
In June 2019, we raised approximately $197.8 million in net proceeds from an underwritten public offering. In December 2019, we entered into a $150 million loan agreement with funds managed by Pharmakon Advisors LP, a leading global life sciences investment firm, and drew down the first tranche of $75 million with the close of the transaction. | ✓ | | In December 2019, we entered into the Syros Collaboration to FASB ASC Topic 718, the amounts shown exclude the impactdiscover, develop and commercialize novel therapies for SCD and beta thalassemia. |
| ✓ | | In 2019, we appointed a chief scientific officer and a chief human resources officer to our senior management team. |
| Overview of estimated forfeituresExecutive Compensation Program |
Executive Compensation Philosophy Our executive compensation program is guided by our overarching philosophy of paying for demonstrable performance. Consistent with this philosophy, we have designed our executive compensation program to achieve the following primary goals: attract, motivate and retaintop-performing senior executives; establish compensation opportunities that are competitive and reward performance; and align the interests of our senior executives with the interests of our stockholders to drive the creation of sustainable long-term value. Executive Compensation Program Design Our executive compensation program is designed to be reasonable and competitive, and balance our goal of attracting, motivating, rewarding and retainingtop-performing senior executives with our goal of aligning their interests with those of our stockholders. The Compensation Committee annually evaluates our executive compensation program to ensure that it is consistent with our short- and long-term goals and the dynamic nature of our business. Our executive compensation program consists of a mix of compensation elements that balance achievement of our short-term goals with our long-term performance. We provide short-term incentive compensation opportunities in the form of annual cash bonuses, which focus on our achievement of annual corporate goals. We also provide long-term incentive compensation opportunities in the form of equity awards, including both stock options and restricted stock units, or RSUs, which focus on our long-term performance. We believe that stock options provide a strong reward for growth in the market price of our common stock because their entire value depends on future stock price appreciation. We believe RSUs also reward growth in the market price of our common stock because they derive additional value from future stock price appreciation, and they are less dilutive to our stockholders because they require fewer shares than stock options. In addition, we believe that the multi-year vesting requirements applicable to both stock options and RSUs encourage retention because our senior executives are incentivized to remain employed through the vesting period. Our executive compensation program is also designed to incorporate sound practices for compensation governance. Below we summarize such practices. What We Do: | ✓ | | Maintain an Independent Compensation Committee. The Compensation Committee consists solely of independent directors. |
| ✓ | | Retain an Independent Compensation Advisor. The Compensation Committee engages its own compensation advisor to provide information and analysis related to service-based vesting conditions. For additional informationannual executive compensation decisions, including the 2019 executive compensation decisions, and other advice on executive compensation independent of management. |
| ✓ | | Review Executive Compensation Annually. The Compensation Committee annually reviews our compensation strategy, including a review and determination of our compensation peer group used for comparative purposes. |
| ✓ | | EmphasizeAt-Risk Compensation. Our executive compensation program is designed so that a significant portion of our executive officers’ compensation is “at risk” based on our corporate performance, as well as equity-based, to align the interests of our executive officers and stockholders. |
| ✓ | | Use aPay-for-Performance Philosophy. The majority of our executive officers’ compensation is directly linked to corporate performance and includes a significant long-term equity component, thereby making a substantial portion of each executive officer’s total compensation dependent upon our stock price and/or total stockholder return. |
| ✓ | | Align Board and Executive with Shareholder Interests. Our executive officers (andnon-executive members of our senior management team), as well as the members of our board of directors, are subject to stock ownership guidelines requiring each of them to maintain ownership of a predetermined amount of company stock. |
| ✓ | | Use Double TriggerChange-in-Control Protection.Change-in-control payments and benefits to our executive officers occur only upon a qualifying termination of employment in connection with a change in control, not merely upon a change in control. |
What We Don’t Do: | ✗ | | No Executive Retirement Plans. We do not offer pension arrangements or retirement plans or arrangements to our executive officers that are different from or in addition to those offered to our other employees. |
| ✗ | | Limited Perquisites. We do not view perquisites as a significant component of our executive compensation program. Accordingly, we do not provide significant perquisites to our executive officers, including our NEOs, except for limited travel stipends or limited housing and travel reimbursements for recruitment and retention purposes. |
| ✗ | | No Special Health and Welfare Benefits. Our executive officers participate in our health and welfare benefits programs on the valuation assumptions underlyingsame basis as our other employees. |
| ✗ | | No Post-Employment Tax Payment Reimbursement. We do not provide any tax reimbursement payments (including“gross-ups”) on anychange-in-control or severance payments or benefits. |
| ✗ | | No Hedging or Pledging Our Equity Securities. We prohibit our executive officers, the valuemembers of restricted stock and options, see Part II, Item 8 “Financial Statements and Supplementary Data” of our 2016 Annual Report onForm 10-K in the Notes to Consolidated Financial Statements, Note 7, “Stock-based awards”. |
(2) | The amounts reported for 2016 reflect the cash incentive compensation determined by our Compensation Committee (for the Named Executive Officers other than the CEO), and by our Board of Directors upon recommendationand certain other employees from hedging or pledging our securities. |
| ✗ | | No Stock OptionRe-Pricing. Our equity plans do not permit stock options to be repriced to a lower exercise or strike price without the approval of our Compensation Committee (for our CEO), based on achievement of certain research and development, clinical, financial and operational metrics related to our 2016 corporate objectives, as specified by our Board of Directors. |
(3) | The amounts reported in this column consist of employer matching contributions received by Dr. Love, Mr. Farrow and Ms. Suvari in connection with the Company’s 401(k) plan benefits, described in greater detail below, and certain termination benefits paid to Dr. Ramos, as described in greater detail below. |
(4) | Includes $507,838 representing the expense recognized by the Company resulting from the modification of 24,821 shares of performance-based restricted stock awards awarded to and purchased by Dr. Love. The performance goals associated with these restricted stock awards were modified and subsequently achieved during 2016. |
(5) | Mr. Farrow joined us in April 2016, and the amount reported in the salary column reflects his partial year of service with us. |
stockholders.(6) | Ms. Suvari joined us in October 2016, and the amount reported in the salary column reflects her partial year of service with us. In addition, Ms. Suvari was paid aone-time cash bonus on January 31, 2017, which we deemed to be compensation earned in 2017 and is not included in the table above. |
(7) | Dr. Ramos’ employment with the Company ended effective October 24, 2016. We entered into a Termination Letter Agreement with Dr. Ramos, effective November 7, 2016, which provided for the payment of cash severance, the continuation of certain benefits, and accelerated vesting for certain of Dr. Ramos’ outstanding stock options. See “Termination Agreement with Eleanor L. Ramos, M.D.”, below, for further details. |
(8) | Includes (a) $165,755 representing the expense recognized by the Company resulting from the modification of 9,286 performance-based stock options awarded to Dr. Ramos and (b) $947,634 resulting from the acceleration of options to purchase an aggregate of 54,999 shares pursuant to the Termination Letter Agreement. |
(9) | Represents cash severance and benefits continuation paid or payable pursuant to the Termination Letter Agreement. |
BaseSalaries.
| “Say-on-Pay” Vote on Executive Compensation |
At our 2019 Annual Meeting of Stockholders, we held anon-binding, advisory vote on the compensation of our NEOs (a“Say-on-Pay” vote) and approximately 97% of the votes cast approved our executive compensation program for 2018. Our Board of Directors and Compensation Committee consider the result of theSay-on-Pay vote in determining the compensation of our executive officers. Based on the strong level of support for our executive compensation program demonstrated by the result of last year’sSay-on-Pay vote, among other factors, the Board of Directors and the Compensation Committee determined not to implement significant changes to our executive compensation program for 2019. The Board of Directors and the Compensation Committee will continue to consider the result of theSay-on-Pay vote, as well as feedback received throughout the year, when making compensation decisions for our executive officers in the future because we value the opinions of our stockholders. In addition, consistent with the recommendation of our Board of Directors and the preference of our stockholders as reflected in thenon-binding, advisory vote on the frequency of futureSay-on-Pay votes held at our 2018 Annual Meeting of Stockholders, we intend to hold an annualSay-on-Pay vote. Our nextSay-on-Pay vote will be held at the Annual Meeting. | Governance of Executive Compensation Program |
Role of the Compensation Committee and the Board of Directors The Compensation Committee discharges many of the responsibilities of our Board of Directors relating to the compensation of our executive officers, including our NEOs. The Compensation Committee oversees and evaluates our compensation and benefits policies generally, and the compensation plans, policies and practices applicable to our CEO and other executive officers. As described below, the Compensation Committee retains a compensation consultant to provide support in its review and assessment of our executive compensation program. In addition, during 2019, pursuant to our Amended and Restated Equity Award Grant Policy, or Equity Award Grant Policy, the Compensation Committee delegated to our CEO the authority to approve grants of equity awards, subject to certain parameters, under the 2015 Plan and any other equity compensation plan that the Compensation Committee or the Board may determine to be subject to the policy, excluding our 2017 Inducement Equity Plan, or 2017 Inducement Plan. In January 2020, our Compensation Committee further amended and restated the Equity Award Grant Policy to provide for the delegation of such authority to a committee comprised of our CEO and at least one other executive officer, and such committee is currently comprised of our CEO and our Chief Human Resources Officer. See “Other Compensation Policies and Practices—Equity Award Grant Policy.” At the beginning of the year, the Compensation Committee reviews and approves the primary elements of compensation—base salary increases, annual cash bonuses, and annual equity awards—for our CEO, and for all individuals at or above the level of Vice President who report directly to our CEO, which includes our other NEOs. In addition, the Compensation Committee may deem it advisable to review and approve subsequent compensation opportunities for our CEO and such other individuals. Compensation-Setting Factors When reviewing and approving the amount of each compensation element and the target total compensation opportunity for our executive officers, the Compensation Committee considers the following factors: our performance against the annual corporate goals established by the Compensation Committee in consultation with management; each executive officer’s skills, experience and qualifications relative to other similarly-situated executives at the companies in our compensation peer group; the scope of each executive officer’s role compared to other similarly-situated executives at the companies in our compensation peer group; the performance of each individual executive officer, based on an assessment of his or her contributions to our overall performance, ability to lead his or her department and work as part of a team, all of which reflect our core values; compensation parity among our executive officers; our financial performance relative to our peers; the compensation practices of our compensation peer group and the positioning of each executive officer’s compensation in a ranking of peer company compensation levels; and the recommendations provided by our CEO with respect to the compensation of our other executive officers. These factors provide the framework for compensation decisions for each of our executive officers, including our NEOs. The Compensation Committee does not assign relative weights or rankings to these factors, and does not consider any single factor as determinative in the compensation of our executive officers. Rather, the Compensation Committee relies on its own knowledge and judgment in assessing these factors and making compensation decisions. Role of Management In discharging its responsibilities, the Compensation Committee works with management, including our CEO. Our management assists the Compensation Committee by providing information on corporate and individual performance, market compensation data and management’s perspective on compensation matters. In addition, at the beginning of each year, our CEO reviews the performance of our other executive officers, including our other NEOs, based on our achievement of our annual corporate goals and each executive officer’s achievement of his or her departmental and individual goals established for the prior year and his or her overall performance during that year. The Compensation Committee solicits and reviews our CEO’s recommendations for base salary increases, annual cash bonuses, annual equity awards and any other compensation opportunities for our other executive officers, including our other NEOs, and considers our CEO’s recommendations in determining such compensation. Role of Compensation Consultant The Compensation Committee engages an external compensation consultant to assist it by providing information, analysis and other advice relating to our executive compensation program. For 2019, the Compensation Committee engaged Compensia, Inc., a national compensation consulting firm, or Compensia, as its compensation consultant to advise on executive compensation matters, including: review and analysis of the compensation for our executive officers, including our NEOs; review and input on the Compensation Discussion and Analysis section of our proxy statement for our 2019 Annual Meeting of Stockholders; research, development and review of our compensation peer group; and support on other compensation matters as requested throughout the year. Compensia reports directly to the Compensation Committee and to the chair of the Compensation Committee. Compensia also coordinates with our management for data collection and job matching for our executive officers. Compensia did not provide any other services to us in 2019. The Compensation Committee has evaluated Compensia’s independence pursuant to the listing standards of the relevant NASDAQ and SEC rules and has determined that no conflict of interest has arisen as a result of the work performed by Compensia. Role of Market Data For purposes of comparing our executive compensation against the competitive market, the Compensation Committee reviews and considers the compensation levels and practices of a group of peer companies. This compensation peer group consists of public biotechnology companies that are similar to us in terms of market capitalization, stage of development, geographical location and number of employees. The Compensation Committee reviews our compensation peer group at least annually and makes adjustments to our peer group if necessary, taking into account changes in both our business and our peer companies’ businesses. In November 2018, the Compensation Committee, with the assistance of Compensia, reviewed our compensation peer group to determine our peer group for the remainder of 2018 and for 2019. The Compensation Committee considered the increases in our market capitalization and our headcount relative to prior periods, and our potential commercial launch, as reflected in the following criteria: publicly-traded companies headquartered in the United States; companies in the biotechnology sector; similar market capitalization—within a range of approximately 0.33x to approximately 3.0x our then-current market capitalization of approximately $2.35 billion (approximately $775 million to approximately $7 billion); the stage of development of each company’s lead candidate (with a preference for companies with Phase 2 or Phase 3 clinical development programs,pre-commercial companies and commercial companies); companies developing either orphan drugs or with a rare disease focus; and similar headcount—within a range of approximately 0.5x to approximately 2.0x our then-current headcount of 148 employees (approximately 75 to 300 employees). Based on a review of the analysis prepared by Compensia, the Compensation Committee approved the updated compensation peer group below for the remainder of 2018 and for 2019. This peer group was used in evaluating the 2019 annual base salary, target annual bonus opportunities and equity awards for our NEOs. | | | | | 2018—2019 Compensation Peer Group | Acceleron Pharma Agios Pharmaceuticals Aimmune Therapeutics Alder BioPharmaceuticals AnaptysBio Arena Pharmaceuticals Atara Biotherapeutics | | bluebird bio Blueprint Medicines Coherus Biosciences Epizyme FibroGen Insmed Loxo Oncology | | Portola Pharmaceuticals Regenxbio Sage Therapeutics Spark Therapeutics Ultragenyx Pharmaceuticals Versartis Xencor |
In July 2019, the Compensation Committee, with the assistance of Compensia, reviewed our compensation peer group to determine our peer group for the remainder of 2019 and for 2020. The Compensation Committee considered our potential estimated revenues in 2020, the increase in our market capitalization and headcount and the near-commercial stage of our lead product candidate, as reflected in the following criteria: publicly-traded companies headquartered in the United States; companies in the biotechnology and pharmaceutical sector; similar estimated revenues in 2020—up to 4.0x our projected 2020 revenue; similar market capitalization—within a range of approximately 0.33x to approximately 3.0x our then-current market capitalization of approximately $3.3 billion (approximately $1.1 billion to approximately $9.9 billion); the stage of development of each company’s lead candidate (with a preference for companies with candidates pending approval, approved or commercialized, and excluding companies whose lead candidates were in Phase 2 or Phase 3 development); companies developing either orphan drugs or with a rare disease focus; and similar headcount—within a range of approximately 0.33x to approximately 3.0x our then-current headcount of 171 employees (approximately 50 to 500 employees). Based on a review of the analysis prepared by Compensia, the Compensation Committee approved the updated compensation peer group below for the remainder of 2019 and for 2020. | | | | | 2019—2020 Compensation Peer Group | ACADIA Pharmaceuticals Acceleron Pharma Agios Pharmaceuticals Aimmune Therapeutics Alnylam Pharmaceuticals Amicus Therapeutics bluebird bio | | Coherus Biosciences Epizyme FibroGen Insmed Intercept Pharmaceuticals Nektar Therapeutics | | Portola Pharmaceuticals Regenxbio Sage Therapeutics Sarepta Therapeutics Spark Therapeutics Ultragenyx Pharmaceuticals |
The Compensation Committee uses market data—from our compensation peer group and from the Radford Global Life Sciences Compensation survey—as one factor in evaluating whether the compensation for our executive officers is competitive in the market. The Compensation Committee also relies on its own knowledge and judgment in evaluating market data and making compensation decisions. | Primary Elements of Executive Compensation Program |
The primary elements of our executive compensation program are: short-term incentive compensation in the form of annual cash bonuses; and long-term incentive compensation in the form of annual equity awards. We do not have a specific policy regarding the percentage allocation between short- and long-term, or fixed and variable, compensation elements. The balance between these components may change from year to year based on corporate strategy, company performance, market forces and company objectives, among other considerations, but consistent with our philosophy of paying for demonstrable performance, our executive compensation program emphasizes variable pay over fixed pay. For example, in 2019, our CEO and other NEOs had the following target pay mix: 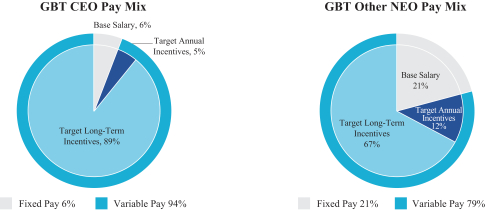
Our executive officers, including our NEOs, are also eligible to participate in our standard employee benefit plans, such as our health and welfare benefits plans, our 2015 Employee Stock Purchase Plan, or ESPP, and our 401(k) Plan on the same basis as our other employees. In addition, as described below, our executive officers, including our NEOs, are entitled to certainchange-in-control severance payments and benefits and certain termination payments and benefits not in connection with a change in control pursuant to our Amended and Restated Severance and Change in Control Policy. Base Salary We pay base salaries to our executive officers, including our NEOs, as the fixed portion of their compensation to provide them with a reasonable degree of financial certainty, and to attract and retaintop-performing individuals. At the time of hire, base salaries are determined for our executive officers, including our NEOs, based on the factors described in “Governance of Executive Compensation Program—Compensation-Setting Factors” above. Typically, at the beginning of each year, the Compensation Committee reviews base salaries for our executive officers, including our NEOs, based on such factors to determine if an increase is appropriate. In addition, base salaries may be adjusted in the event of a promotion or significant change in responsibilities. 2019 Annual Base Salary In January 2019, the Compensation Committee reviewed the base salaries of our executive officers, including our namedNEOs. The Compensation Committee considered the factors described in “Governance of Executive Compensation Program—Compensation-Setting Factors” above. In particular, the Compensation Committee considered better alignment with comparable positions from our compensation peer group in determining the larger base salary increases for Dr. Love and Dr. Lehrer-Graiwer. Effective in February 2019, the Compensation Committee approved the base salaries of our NEOs, except Dr. Cathers, below. | | | | | | | | | | | | | NEO | | 2018 Annual
Base Salary | | | 2019 Annual
Base Salary | | | Percentage
Increase | | | | | | Dr. Love | | $ | 575,000 | | | $ | 600,000 | | | | 4.3 | % | | | | | Mr. Farrow | | $ | 427,500 | | | $ | 442,500 | | | | 3.5 | % | | | | | Mr. Johnson | | $ | 440,000 | | | $ | 455,000 | | | | 3.4 | % | | | | | Dr. Lehrer-Graiwer | | $ | 400,000 | | | $ | 425,000 | (1) | | | 6.3 | % |
| (1) | In connection with his promotion to Chief Medical Officer and additional responsibilities in October 2019, Dr. Lehrer-Graiwer’s 2019 annual base salary was increased from $425,000 to $460,000, effective October 1, 2019. |
In February 2019, we hired Dr. Cathers as our Chief Scientific Officer. The Compensation Committee considered the factors described in “Governance of Executive Compensation Program-Compensation-Setting Factors” above, particularly market data from our compensation peer group for comparable positions, in approving Dr. Cathers’ annual base salary of $375,000. The actual base salaries paid to our NEOs in 2019 are set forth in the “Summary Compensation Table” below. Short-Term Incentive Compensation Annual Cash Bonuses We provide short-term incentive compensation opportunities to our executive officers, from time to time and makes adjustments (or,including our NEOs, in the caseform of annual cash bonuses to drive our Chief Executive Officer, may recommend adjustmentsshort-term success. Our annual cash bonuses for approval by2019 were tied to the Boardachievement of Directors) as it determinesannual corporate and individual performance goals pursuant to be reasonable and necessary to reflect the scope of the executive officer’s performance, contributions, responsibilities, experience, prior salary level, position (in the case of a promotion) and market conditions, including base salary amounts relative to similarly situated executive officers at peer group companies. CashIncentiveCompensation. In January 2016, the Board of Directors adopted the Company’sour Senior Executive Cash Incentive Bonus Plan, (the “Incentive Plan”), which applies to certain key executives (the “Covered Executives”), that are recommended byor Cash Incentive Plan.
Corporate and Individual Performance Goals At the beginning of each year, the Compensation Committee, after reviewing management’s proposal, establishes the annual corporate performance goals that it believes will be the most significant drivers of our short- and selected bylong-term success. The corporate performance goals include target achievement dates based on calendar quarters. Each corporate performance goal has a percentage weighting, and may include an additional percentage weighting for overachievement, based on the BoardCompensation Committee’s assessment of Directors.the goal’s relative significance. In addition, at the beginning of each year, our CEO, in consultation with each of the other executive officers, establishes individual performance goals for each of the other executive officers, including our other NEOs. The Incentive Plan providesindividual performance goals are generally designed to align the goals of our executive officers, including our NEOs, and his or her department with the corporate goals. The Compensation Committee weights annual cash bonuses for each of our executive officers, including our NEOs, between the achievement of corporate and individual goals. This weighting is the same for each of our executive officers, including our NEOs, who are at the same level. Our CEO does not have individual goals. Rather, his annual cash bonus paymentsis based upon100% on achievement of our corporate goals in recognition of his overall responsibility for our corporate performance. At the attainmentbeginning of the year after the corporate performance objectivesgoals are established, by the Compensation Committee, and related to operational and financial metrics with respect to the Company or any of its subsidiaries (the “Corporate Performance Goals”), which may includeafter reviewing management’s self-assessment, evaluates our achievement of specified researchthe prior year’s corporate performance goals, and development, publication, clinical and/or regulatory milestones, total shareholder return, earnings before interest, taxes, depreciation and amortization, net income (loss) (either before or after interest, taxes, depreciation, stock compensation expense, restructuring charges and/or amortization), changesour overall success in the market priceprior year, and determines our total percentage achievement level. Our CEO evaluates the other executive officers’, including the other NEOs’, achievement of their prior year’s individual performance goals, and makes recommendations for total percentage achievement level. The Compensation Committee considers our CEO’s recommendations, and independently reviews and approves the total percentage achievement level for each of the Company’s common stock, economic value-added, funds from operations or similar measure, sales or revenue, acquisitions or strategic transactions, operating income (loss), cash flow (including, but not limitedother executive officers, including our other NEOs. Target Annual Bonuses The target annual bonus is determined for each of our executive officers, including our NEOs, at the time of hire as well as at the beginning of each year, by reference to operating cash flow and free cash flow), return on capital, assets, equity, or investment, return on sales, gross or net profit levels, productivity, expense, margins, operating efficiency, customer satisfaction, working capital, earnings (loss) per share of the Company’s common stock; bookings, new bookings or renewals; sales or market shares; number of customers, number of new customers or customer references; operating income and/or net annual recurring revenue. Any bonuses paid under thethen applicable Cash Incentive Plan, will be based upon objectively determinable bonus formulas that tie suchwhich sets out the target annual bonuses to one or more performance targets relating tofor employees by position level. In approving the Corporate Performance Goals. The bonus formulas will be adopted in each performance period byCash Incentive Plan, the Compensation Committee considers the factors described in “Governance of Executive Compensation Program—Compensation-Setting Factors” above, with an emphasis on market data from our compensation peer group for comparable positions. Target annual bonuses are the same for executive officers, including our NEOs, who are at the same level, and communicatedrepresent a specific percentage of annual base salary. Annual Cash Bonus Formula The Compensation Committee uses the following formula to calculate annual cash bonuses for each Covered Executive. No bonuses will be paid under the Incentive Plan unlessof our executive officers, including our NEOs: | | | | | | | | | | | | | | | | | | | | | | | | | | ( | | Total % achievement of annual corporate performance goals | | x | | % weighting of annual corporate performance goals | | + | | Total % achievement of annual individual performance goals | | x | | % weighting of annual individual performance goals | | ) | | x | | Target Annual Bonus % | | x | | Annual Base Salary |
2019 Corporate Performance Goals In January 2019, our Board of Directors approved our 2019 annual corporate performance goals and untilweightings as set forth below. | | | | | Category | | Corporate Goal | | Weighting | Oxbryta | | • NDA accepted by FDA • Achieve certain data publication goals • Achieve certain market and commercial launch goals | | 45% (subject to increase to 67.5% upon achievement of a specified stretch goal) 10% (subject to increase to 15% upon achievement of a specified stretch goal) 20% | | | | Inclacumab | | • Achieve certain research goal with respect to inclacumab | | 10% | | | | Pipeline | | • Nominate one candidate for development and achieve certain business development goals | | 10% | | | | Corporate | | • Complete the year with a cash reserve of at least a certain minimum | | 5% |
2019 Target Annual Bonus In January 2019, the Compensation Committee makes a determinationreviewed the target annual bonuses of our executive officers, including our NEOs. The Compensation Committee considered the factors described in “Governance of Executive Compensation Program—Compensation-Setting Factors” above, particularly market data from the companies in our compensation peer group, and approved the 2019 target annual bonuses of our NEOs, except Dr. Cathers, below. | | | | | | | | | NEO | | 2018 Target Annual Bonus | | | 2019 Target Annual Bonus | | Dr. Love | | | 60 | % | | | 60 | % | Mr. Farrow | | | 40 | % | | | 40 | % | Mr. Johnson | | | 40 | % | | | 40 | % | Dr. Lehrer-Graiwer | | | 35 | % | | | 40 | %(1) |
| (1) | In connection with his promotion to Chief Medical Officer and additional responsibilities in October 2019, Dr. Lehrer-Graiwer’s 2019 target annual bonus was increased from 35% to 40% of his annual base salary, effective October 1, 2019. |
In February 2019, we hired Dr. Cathers as our Chief Scientific Officer. The Compensation Committee considered the factors described in “Governance of Executive Compensation Program—Compensation-Setting Factors” above, particularly market data from our compensation peer group for comparable positions and alignment with respect toour other executive officers at the attainmentsame level, in approving Dr. Cathers’ 2019 target annual bonus of 40%. 2019 Annual Cash Bonuses In January 2020, the Compensation Committee evaluated our achievement of the 2019 corporate performance objectives. Notwithstandinggoals. The Compensation Committee considered whether we had achieved each goal, the foregoing,weighting established for each goal, including the weighting for overachievement, management’s self-assessment, and our overall corporate performance in 2019. Based on these considerations, the Compensation Committee approved a 150% achievement level of the 2019 corporate performance goals due in part to certain extraordinary achievements, including: (i) our receipt of approval by the FDA for Oxbryta three months in advance of its PDUFA target action date, (ii) the successful acceleration of our sales force hiring and commercial launch readiness, (iii) our completion of our strategic Syros Collaboration in furtherance of our pipeline and (iv) our success in exceeding our 2019year-end cash goal with our equity and debt financings. The Compensation Committee also reviewed the 2019 individual performance of each of our executive officers, other than our CEO, based on an evaluation conducted by our CEO of their performance against their 2019 individual performance goals. The Compensation Committee approved an achievement level of 100% of the 2019 individual performance goals for each of our NEOs. The table below sets forth the target annual cash bonuses, the relative weighting of corporate and individual performance, the actual achievement level for corporate and individual performance and the 2019 annual cash bonuses earned by our NEOs. | | | | | | | | | | | | | | | | | | | | | | | | | NEO | | 2019 Annual
Base Salary
($) | | | Target Annual
Cash Bonus
(% of annual
base salary) | | | Weighting
(corporate/
individual
performance)
(%) | | | Corporate
Performance
(%) | | | Individual
Performance
(%) | | | Annual Cash
Bonus
($) | | Dr. Love | | $ | 600,000 | | | | 60 | % | | | 100%/0 | | | | 150 | % | | | N/A | | | $ | 540,000 | | Mr. Farrow(1) | | $ | 442,500 | | | | 40 | % | | | 75%/25% | | | | 150 | % | | | 100 | % | | $ | 244,000 | | Dr. Cathers (1)(2) | | $ | 375,000 | | | | 40 | % | | | 75%/25% | | | | 150 | % | | | 100 | % | | $ | 172,000 | | Mr. Johnson(1) | | $ | 455,000 | | | | 40 | % | | | 75%/25% | | | | 150 | % | | | 100 | % | | $ | 250,000 | | Dr. Lehrer-Graiwer | | $ | 460,000 | | | | 40 | % | | | 75%/25% | | | | 150 | % | | | 100 | % | | $ | 253,000 | |
| (1) | Annual cash bonus was immaterially adjusted for rounding. |
| (2) | Dr. Cathers’ employment commencement date was February 25, 2019, and his 2019 annual cash bonus was prorated based on his employment with us for approximately ten months of 2019. |
The annual cash bonuses earned by each of our NEOs for 2019 are set forth in the “Summary Compensation Table” below. Long-Term Incentive Compensation We view long-term incentive compensation in the form of equity awards as an important element of our executive compensation program. The value of equity awards is directly related to stock price appreciation over time, which incentivizes our executive officers to achieve long-term corporate goals and create long-term value for our stockholders. Equity awards also help us attract and retaintop-performing executive officers in a competitive market. At the time of hire, equity awards are granted to our executive officers, including our NEOs, based on the factors described in “Governance of Executive Compensation Program—Compensation-Setting Factors” above. Typically, at the beginning of each year, the Compensation Committee reviews the equity awards for our executive officers, including our NEOs, and determines the size and relative weighting of the annual equity awards it deems reasonable and appropriate based on such factors. The size and relative weighting is the same for each of our executive officers, including our NEOs, who are at the same level. In addition, the Compensation Committee may deem it advisable to grant subsequent equity awards to our executive officers, including our NEOs, and may adjust bonuses payable undertheir equity awards in the Incentive Plan based on achievementevent of individual performance goalsa promotion or pay bonuses (including, without limitation, discretionary bonuses) to Covered Executives under the Incentive Plan based on individual performance goals and/or upon such other terms and conditions assignificant change in responsibilities. 2019 Equity Awards 2019 Annual Equity Awards In January 2019, the Compensation Committee mayconsidered the factors described in its discretion determine.“Governance of Executive Compensation Program—Compensation-Setting Factors” above, particularly market data from the companies in our compensation peer group, and approved the 2019 annual equity awards for our NEOs, except Dr. Cathers, below. EquityIncentiveCompensation. Historically, we have generally granted
| | | | | | | | | NEO | | Stock Options
(Number of Shares) | | | Time-Based RSUs
(Number of Shares) | | Dr. Love | | | 145,000 | | | | 90,000 | | Mr. Farrow | | | 40,000 | | | | 25,000 | | Mr. Johnson | | | 40,000 | | | | 25,000 | | Dr. Lehrer-Graiwer | | | 35,000 | | | | 23,500 | |
The stock options vest, and become exercisable, over a four-year period, with 1/16th of the underlying shares vesting on a quarterly basis (every three months) after the vesting commencement date of February 1, 2019, so that all of the underlying shares will be vested on the date four years after the vesting commencement date, so long as the NEO remains an employee or other service provider (including a consultant) of the Company on such vesting dates. The time-based RSUs vest over a four-year period, with 1/8th of the underlying shares vesting on a semi-annual basis (every six months) after the vesting commencement date of February 1, 2019, so that all of the underlying shares will be vested on the date four years after the vesting commencement date, so long as the NEO remains an employee or other service provider (including a consultant) of the Company on such vesting dates. 2019 Equity Awards for New Executive Officers In February 2019, we hired Dr. Cathers as our Chief Scientific Officer. The Compensation Committee considered the factors described in “Governance of Executive Compensation Program—Compensation-Setting Factors” above, particularly market data from our compensation peer group for comparable positions, in approving Dr. Cathers’ new hire equity awards as follows: a stock option to purchase 45,000 shares of our common stock and 30,000 time-based RSUs. The stock options vest, and become exercisable, over a four-year period, with 1/4th of the underlying shares vesting on the first anniversary of the vesting commencement date of February 25, 2019, and thereafter, 1/12th of the remaining underlying shares vest on a quarterly basis so that all of the underlying shares will be vested on the date four years after the vesting commencement date, so long as Dr. Cathers remains an employee or other service provider (including a consultant) of the Company on such vesting dates. The time-based RSUs vest over a four-year period, with 1/4th of the underlying shares vesting on the first anniversary of the vesting commencement date of March 1, 2019, and thereafter 1/6th of the remaining underlying shares vest on a semi-annual basis (every six months) so that all of the underlying shares will be vested on the date four years after the vesting commencement date, so long as Dr. Cathers remains an employee or other service provider (including a consultant) of the Company on such vesting dates. In connection with his appointment as our Chief Medical Officer in October 2019, the Compensation Committee additionally granted Dr. Lehrer-Graiwer an option to purchase 5,000 shares of our common stock and time-based RSUs that may vest and be settled for 1,500 shares of our common stock. The option vests, and becomes exercisable, over a four-year period, with 1/16th of the underlying shares vesting on a quarterly basis (every three months) after the vesting commencement date of October 1, 2019, so that all of the underlying shares will be vested on the date four years after the vesting commencement date, so long as Dr. Lehrer-Graiwer remains an employee or other service provider (including a consultant) of the Company on such vesting dates. The time-based RSUs vest over a four-year period, with 1/8th of the underlying shares vesting on a semi-annual basis (every six months) after the vesting commencement date of October 1, 2019, so that all of the underlying shares will be vested on the date four years after the vesting commencement date, so long as Dr. Lehrer-Graiwer remains an employee or other service provider (including a consultant) of the Company on such vesting dates. As noted above, Dr. Lehrer-Graiwer resigned from the company effective April 17, 2020, and did not remain in our service (including as a consultant) past that date. The equity awards granted to our employees,NEOs in 2019 are set forth in the “Summary Compensation Table” and the “Grants of Plan-Based Awards for Fiscal Year 2019” table below. Health and Welfare Benefits Our executive officers, including our namedNEOs, are eligible to participate in the same employee benefit plans that are generally available to all of our employees, subject to the satisfaction of certain eligibility requirements, such as medical, dental, and life and disability insurance plans. We pay, on behalf of our employees, all or a portion of the premiums for health, life and disability insurance. 2015 Employee Stock Purchase Plan Our executive officers, including our NEOs, are eligible to participate in connectionour ESPP on the same basis as our other full-time employees. The ESPP permits eligible employees to set aside a portion of their compensation during offering periods that are generally two years long, with their initial employment with us. Beginning in 2017, our practice ispurchase periods generally every six months during each offering period, and use such contributions to grant a combination of stock options and restricted stock units to our employees, including executive officers, in connection with their initial employment with us. Prior to our initial public offering in August 2015, we granted to employees, including certainpurchase shares of our named executive officers,common stock at their election, sharesa purchase price equal to 85% of restricted stock purchased atthe lower of the fair market value as determined by our Board of Directors at the timeshares on the first business day of grant. We also have historically granted stock options and prior to our initial publicthe offering inperiod or the caselast business day of certain of our namedthe purchase period.
401(k) Savings Plan Our U.S. executive officers, at their election, shares of restricted stock purchased at fair market value, as determined by our Board of Directors at the time of grant on an annual basis as part of annual performance reviews of our employees. Beginning in 2017, our equity award grant policy also contemplates the grant of stock options and restricted stock units to existing employees, including our named executive officers,NEOs, are eligible to participate in connection with annual performance evaluations. Outstanding Equity Awards at FiscalYear-End
The following table sets forth certain information with respect to outstanding equity awards held by each of our named executive officers as of December 31, 2016.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Option Awards | | | Stock Awards | | Name | | Number of
Securities
Underlying
Unexercised
Options (#)
Exercisable | | | Number of
Securities
Underlying
Unexercised
Options (#)
Unexercisable
(1) | | | Equity
Incentive
Plan
Awards:
Number of
Securities
Underlying
Unexercised
Unearned
Options
(#)(1) | | | Option
Exercise
Price
($) | | | Option
Expiration
Date | | | Number
of shares
of stock
that
have not
vested
(#)(1) | | | Market
value of
shares of
stock that
have not
vested
($)(2) | | | Equity
Incentive
Plan
Awards:
Number of
unearned
shares or
options
that have
not vested
(#) | | | Equity
Incentive
Plan
Awards:
Market
value or
payout
value of
unearned
shares or
options
that have
not
vested
($)(3) | | | (a) | | (b) | | | (c) | | | (d) | | | (e) | | | (f) | | | (g) | | | (h) | | | (i) | | | (j) | | Ted W. Love, M.D. | | | — | | | | — | | | | — | | | | — | | | | — | | | | 4,019 | (4) | | | 58,075 | | | | — | | | | — | | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 267,856 | (5) | | | 3,870,519 | | | | — | | | | — | | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 125,890 | (6) | | | 1,819,111 | | | | — | | | | — | | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 24,821 | (7) | | | 358,663 | | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 99,285 | (8) | | | 1,434,668 | | | | | | | | | | | | Jeffrey Farrow | | | — | | | | 120,000 | (9) | | | — | | | $ | 14.96 | | | | 2/24/2026 | | | | — | | | | — | | | | — | | | | — | | | | | | | | | | | | Tricia Suvari | | | — | | | | 100,000 | (10) | | | — | | | $ | 18.25 | | | | 10/11/2026 | | | | — | | | | — | | | | — | | | | — | | | | | | | | | | | | Eleanor Ramos(11) | | | 47,500 | | | | — | | | | — | | | $ | 3.40 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | | 135,356 | | | | — | | | | — | | | $ | 0.49 | | | | — | | | | — | | | | — | | | | — | | | | — | |
(1) | All of the equity awards held by our named executive officers will accelerate and become fully vested and exercisable ornon-forfeitable if the equity holder is subject to an involuntary termination within 12 months after a sale event. The vesting acceleration of the equity awards held by our named executive officers is described in greater detail in “Employment Arrangements with Our Named Executive Officers—Change in Control Policy”. |
(2) | Computed in accordance with SEC rules as the number of unvested shares multiplied by the closing market price of our common stock on December 31, 2016, which was $14.45. The actual value (if any) to be realized by the officer depends on whether the shares vest and the future performance of our common stock. |
(3) | Computed in accordance with SEC rules as the number of unvested shares multiplied by the closing market price of our common stock at December 31, 2016, which was $14.45. The actual value (if any) to be realized by the officer depends on whether the performance milestones related thereto are achieved, whether the shares vest following achievement of the performance milestones, and the future performance of our common stock. |
(4) | Dr. Love purchased 21,428 shares of our restricted common stock under our 2012 Stock Option and Grant Plan (the “2012 Plan”) on September 4, 2013 in connection with joining our Board of Directors. Of this total, 25% of the shares of restricted common stock vested on September 4, 2014, and the remaining shares vest quarterly over the following three years thereafter, subject to Dr. Love’s continuous service through each such vesting date. |
(5) | Dr. Love purchased 714,285 shares of our restricted common stock under our 2012 Plan on June 11, 2014 in connection with the commencement of his employment as CEO. Of this total, 25% of the shares of restricted common stock vested on June 11, 2015, and the remaining shares vest quarterly over the following three years thereafter, subject to Dr. Love’s continuous service through each such vesting date. |
(6) | Dr. Love purchased 201,428 shares of our restricted common stock under our 2012 Plan on April 9, 2015 in connection with an annual replenishment grant. Of this total, 1/16th of the shares of restricted common stock vest on a quarterly basis from the vesting commencement date of April 9, 2015, such that all of the shares will be fully vested on April 9, 2019, provided Dr. Love remains in continuous service through each such vesting date. |
(7) | Dr. Love purchased 99,285 shares of our restricted common stock under our 2012 Plan on April 9, 2015. Of this total, 25% of the shares are subject to vesting upon the achievement of each of four (4) specified corporate operating milestones on or before certain specified dates, subject to Dr. Love’s continuous service through each such vesting date. On March 10, 2016 and September 29, 2016, the Compensation Committee determined that two of the four corporate milestones were met within the specified timeline and accordingly 49,643 shares of the restricted common stock in column (i) vested. In addition, one of the four corporate milestones was not met within the specified timeline and accordingly, 24,821 shares of the restricted common stock were cancelled during 2016. |
(8) | Dr. Love purchased 99,285 shares of our restricted common stock under our 2012 Plan on April 9, 2015. The shares will not vest until our market capitalization (determined based on the number of shares of common stock outstanding multiplied by the closing market price for our common stock as reported on NASDAQ) exceeds at least $2.0 billion for 20 consecutive trading days on or before the date twenty-four (24) months after the closing of our initial public offering (IPO), which was on August 11, 2015. |
(9) | Mr. Farrow received a grant of an option to purchase 120,000 shares of our common stock under our 2015 Stock Option and Incentive Plan (the “2015 Plan”) on February 24, 2016 in connection with the commencement of his service relationship with the Company, as an advisor. His full time employment with the Company commenced effective April 4, 2017. Of this total, 25% of the shares subject to the option vested on April 4, 2017, and the remaining shares vest quarterly over the following three years thereafter, subject to Mr. Farrow’s continuous service through each such vesting date. |
(10) | Ms. Suvari received a grant of an option to purchase 100,000 shares of our Common Stock under our 2015 Plan on October 11, 2016 in connection with the commencement of her employment. 25% of the shares subject to the option will vest on October 11, 2017, and the remaining shares vest quarterly over the following three years thereafter, subject to Ms. Suvari’s continuous service through each such vesting date. |
(11) | Dr. Ramos’ employment ended effective October 24, 2016. As of December 31, 2016, Dr. Ramos held outstanding stock options to purchase a total of 182,856 shares of common stock, and was entitled to exercise these stock options until ninety (90) days after her October 24, 2016 separation date under the terms of her Termination Letter Agreement with us. |
401(k) Savings Plan and Other Benefits
We maintain atax-qualified retirement plan, or 401(k) Plan, thaton the same basis as our other employees. The 401(k) Plan provides eligible U.S. employees with an opportunity to save for retirement on a tax advantaged basis. Eligible employees are able to defer eligible compensation subject to applicable annual limits of the Internal Revenue Code of 1986, as amended, (the “Code”) limits.or the Code. Employees’pre-tax contributions are allocated to each participant’s individual account and are then invested in selected investment alternatives according to the participants’ directions. Employees are immediately and fully vested in their contributions. Our 401(k) Plan is intended to be qualified under Section 401(a) of the Code with our 401(k) Plan’s related trust intended to be tax exempt under Section 501(a) of the Code. As atax-qualified retirement plan, contributions to our 401(k) Plan and earnings and matching amounts on those contributions are not taxable to the employees until distributed from our 401(k) Plan. In
Since December 2015, ourthe Compensation Committee has approved avarious matching contributions under the 401(k) planPlan. Under our current matching policy under which,approved by the Compensation Committee, effective as of January 1, 2016, subject to reassessment of the cap on matching for the calendar year thereafter,2018, we match in cash 100% of employee’s 401(k) contributions up to the first $500 and, thereafter, we match in cash 50% of employee’s first 6% of 401(k) contributions, subject to a cap of $2,500 per employee. In January 2017, our Compensation Committee approved a revised and simplified 401(k) Plan matching policy under which, effective as of January 1, 2017, subject to reassessment of the cap on matching for the calendar year thereafter, we match in cash 50% ofan employee’s 401(k) contributions, subject to aan annual cap of $2,500$5,000 per employee. We also pay, on behalf Perquisites Perquisites or other personal benefits are not a significant component of our employees,executive compensation program. Accordingly, we do not provide significant perquisites or other personal benefits to our executive officers, including our NEOs, except for limited travel stipends or limited housing and travel reimbursements for one NEO and certain other executive officers for recruitment and retention purposes. During 2019, none of our NEOs received perquisites or other personal benefits that were, in the premiumsaggregate, $10,000 or more for health, lifeeach individual, except Dr. Cathers, for whom we provided a travel allowance of $10,000 per month to cover travel between his residence and disability insurance.our corporate offices in South San Francisco. Employment Arrangements with Our Named Executive Officers
| Employment Arrangements with our NEOs |
Change in Control PolicyPost-Employment Compensation
We consider it essential to the best interests of our stockholders to foster the continuous employment of our key management personnel. In this regard,Accordingly, we recognizebelieve that reasonable and competitive post-employment compensation arrangements are an important part of an executive compensation program to attract and retain highly-qualified senior executives. While the Compensation Committee does not consider the specific amounts payable under these post-employment compensation arrangements when determining the annual compensation of our NEOs, we believe that providing our executives with post-employment payments and benefits in connection with a change in control are in the best interests of our stockholders because the possibility of a change in control and the related uncertainty may exist and that the uncertainty and questions that it may raise among management could result inlead to the departure or distraction of management personnelsenior executives to the detriment of the Companyour company and stockholders. In July 2015, our stockholders. In orderBoard of Directors adopted a change in control policy, which applies to our executive officers, including our NEOs, to reinforce and encourage the continued attention and dedication of certain key memberssenior executives in the event of management,a change in July 2015,control by providing these executives with certain cash payments, equity acceleration and other benefits upon a qualifying termination event in connection with a change in control. Additionally, prior to the January 2020 amendment of our Boardchange in control policy (which amendment is in the form of Directors adopted aour current Amended and Restated Severance and Change in Control Policy, whichas described below), our CEO was amendedentitled to certain post-employment compensation upon a qualifying termination event independent of any change in January 2016 (the “Policy”)control pursuant to the terms of his employment offer letter with us, as further described below under “Employment Offer Letters—CEO.” Pursuant to the Policy,our change in control policy, in the event the employment of any of our named executive officersNEOs is terminated by us or our acquirer or successor without Cause or an NEO resigns for Good Reason (as such terms are defined in the Policy)our change in control policy), in either case, within one year after the consummation of a saleSale Event (as defined in the 2015 Plan) (suchone-year period, the “Change in Control Period”), he or she will be entitled to receive the following payments and benefits, or CIC Benefits, subject to his or her execution andnon-revocation of a severance agreement within 60 days following the date of such termination, including a general release of claims: a lump sum cash payment equal to 12 months (or 18 months in the case of our CEO) of the NEO’s then-current base salary; a lump sum cash payment equal to 100% of the NEO’s target annual bonus for the year in which the closing of the Sale Event occurred, which, under our Amended and Restated Severance and Change in Control Policy, was increased from 100% to 150% in the case of our CEO; a lump sum cash payment equal to the prorated annual cash bonus payout of the NEO for the portion of the year in which the closing of the Sale Event occurred, based on the NEO’s annual cash bonus target and the date of termination of his or her employment or other service relationship with the company; if the NEO elects to continue his or her group healthcare benefits, a cash payment of an amount equal to the monthly employer contribution we would have made to provide the NEO with health insurance if | he or she had remained employed by us until the earlier of (i) 12 months (or 18 months in the case of our CEO) following the date of termination, or (ii) the end of the NEO’s COBRA health continuation period; and |
full acceleration of vesting of all outstanding equity awards under the 2015 Plan, the 2017 Inducement Plan and such additional equity incentive plans and arrangements as may be applicable from time to time, including all performance-based equity awards, which will accelerate and vest based on the deemed achievement of 100% of target levels as of the date of the NEO’s termination. As described above, the CIC Benefits are “double trigger” because the change in control alone does not trigger such payments and benefits. Rather, the CIC Benefits are triggered only if there is a qualifying termination of an NEO’s employment within the Change in Control Period. In the case of the acceleration of vesting of outstanding equity awards, we use this double-trigger arrangement to protect against the loss of retention value following a change in control of the Company and to avoid windfalls, both of which could occur if vesting of either equity or cash-based awards accelerated automatically as a result of the transaction. In addition, upon a Sale Event, to the extent Section 280G of the Internal Revenue Code of 1986, as amended, or Section 280G, is applicable, each NEO who is then employed with us will be entitled to receive the better treatment of: (i) payment of the full amounts set forth above to which the NEO is entitled or (ii) payment of such lesser amount that does not trigger excise taxes under Section 280G. None of our NEOs are entitled to excise tax payments (or“gross-ups”) relating to a change in control of the Company. The payments and benefits provided under our change in control policy are designed to be competitive in the market. Accordingly, in January 2020, following its review of our then current change in control policy compared to the post-employment compensation arrangements of the companies in our compensation peer group, the Compensation Committee determined that the policy was in need of enhancement to recruit and retain top talent and to align with market norms. As a result of this review, the Compensation Committee approved our Amended and Restated Severance and Change in Control Policy, which, in addition to the above described benefits, provides our NEOs with certain payments and benefits upon a qualified termination event outside of the Change in Control Period. Pursuant to the Amended and Restated Severance and Change in Control Policy, in the event the employment of any of our NEOs is terminated by us or our acquirer or successor without Cause or an NEO resigns for Good Reason outside of the Change in Control Period, he or she will be entitled to receive the following payments and benefits, subject to his or her execution andnon-revocation of a severance agreement within 60 days following the date of such termination, including a general release of claims: a lump sum cash payment equal to nine months (or 12 months of the NEO’s then-current base salary; in the case of Dr. Love as our CEO)CEO only, a lump sum cash payment equal to (i) 100% of the named executive officer’s then-current base salary;CEO’s target annual bonus for the year in which the termination of his employment or other service relationship with the company occurred, plus (ii) a prorated annual cash bonus payout for the portion of the year in which the termination of his employment or other service relationship with the company occurred, based on the CEO’s annual cash bonus target and the date of termination of his employment or other service relationship with the company; and payment of the named executive officer’s target annual incentive compensation;
if the named executive officerNEO elects to continue his or her group healthcare benefits, a cash payment of an amount equal to the monthly employer contribution we would have made to provide the named executive officerNEO with health insurance if he or she had remained employed by us until the earlier of (i) nine months (or 12 months in the case of Dr. Love as our CEO) following the date of termination, or (ii) the end of the named executive officer’sNEO’s COBRA health continuation period;period. For an estimate of the potential payments and all time-based stock options benefits that our NEOs would have been eligible to receive under the Amended and Restated Severance and Change in Control Policy if a hypothetical change in control or other stock-basedtrigger event had occurred on December 31, 2019, see “Potential Payments on Termination or Change in Control” below.
Employment Offer Letters NEOs We have entered into a written employment offer letter with each of our NEOs. These offer letters set forth the basic terms and conditions of employment, including initial base salary, eligibility to participate in the Cash Incentive Plan, initial equity awards, grantedeligibility to participate in our standard employee benefits plans and theat-will employment relationship. These offer letters also require that each NEO execute our standard employee confidentiality and assignment agreement. Prior to January 2020, our NEOs were also eligible to receive certain severance and/orchange-in-control payments and benefits in accordance with our change in control policy (and pursuant to his offer letter, for our CEO) as described in “Post-Employment Compensation” above. As of January 2020, our NEOs are eligible to receive certain severance andchange-in-control payments and benefits in accordance with the Amended and Restated Severance and Change in Control Policy as described in “Post-Employment Compensation” above. CEO Prior to the named executive officer will become fully exercisableadoption of our Amended andnon-forfeitable Restated Severance and all performance-based awards will accelerateChange in Control Policy, Dr. Love’s written employment offer letter provided for certain termination payments and vest based onbenefits not in connection with a change in control. In particular, Dr. Love’s employment offer letter provided that if Dr. Love’s employment were terminated by us without Cause (as defined in the deemed achievement of 100% of target levels as of the date of the named executive officer’s termination. In addition, uponletter) not in connection with a sale event, to the extent Section 280G of the Internal Revenue Code of 1986, as amended, is applicable, each named executive officer who is then employed with us willchange in control, he would be entitled to receive the better treatment of: (i) payment of the full amounts set forth above to which the named executive officer is entitled or (ii) payment of such lesser amount that does not trigger excise taxes under Section 280G.
Employees who are party to an agreement or an arrangement with the Company that provides greater benefits in the aggregate than set forth in the Policy are not eligible to receive anyfollowing payments or benefits under the Policy.
In addition, we have also entered into a written employment agreement with each of our named executive officers that provides for other compensation and benefits, as described below.
Ted W. Love, M.D.
We entered into anat-will employment offer letter agreement with Dr. Love in May 2014, pursuant to which he began serving as our President and CEO in June 2014 (the “CEO Employment Agreement”). Pursuant to the terms of the CEO Employment Agreement, Dr. Love is entitled to an annual base salary currently set at $525,000, subject to adjustment pursuant tohis execution andnon-revocation of a severance agreement, including a general release of claims, his resignation from all positions with us and compliance with our employee compensation policies in effect from time to time. Pursuant to the terms of the CEO Employment Agreement and the Incentive Plan, Dr. Love is eligible to receive annual incentive compensation at a target percentage (currently 60%)instructions regarding Company property:
continuation of his then-current annual base salary, as determined by our Board of Directors. In connection with his appointment as President and CEO, we issued Dr. Love 714,285 shares of our common stock pursuant to a restricted stock award agreement dated June 11, 2014. Dr. Love’s employment under the CEO Employment Agreement isat-will. In the event that Dr. Love’s employment is terminated by the Company without cause, we will provide certain termination benefits, including (i) continuation of base salary for a period of nine months after the effective date ofhis termination date; and (ii)
continuation of group health plan benefits to the extent authorized by and consistent with COBRA, with the cost of the regular premium for such benefits shared in the same relative proportion by us and Dr. Love as in effect on the effectivehis termination date of termination until the earlier of (x)(i) the date that is nine months after the effectivehis termination date, of termination and (y)(ii) the date Dr. Love becomes eligible for health benefits through another employer or otherwise becomes ineligible for COBRA,COBRA. These payments and benefits were designed to be competitive in the market. As noted above under “Post-Employment Compensation,” following its review of the post-employment compensation arrangements of the companies in our peer compensation group, the Compensation Committee adopted the Amended and Restated Severance and Change in Control Policy in January 2020. The post-employment payments and benefits to which Dr. Love is entitled under such recently adopted policy both during and outside of a Change in Control Period supersede and replace those under Dr. Love’s employment offer letter. | Other Compensation Policies and Practices |
Equity Award Grant Policy We have adopted an Amended and Restated Equity Award Grant Policy in January 2020 that sets forth the process and timing for us to follow when we grant equity awards for shares of our common stock to our employees, including our executive officers, or advisors or consultants to us pursuant to any of our equity compensation plans. Pursuant to the policy, all grants of equity awards must be approved in advance by our Board of Directors, the Compensation Committee or, subject to the delegation requirements in the policy, a committee comprised of the CEO and at least one other executive officer of the Company, or Equity Grant Committee. The Equity Grant Committee is currently comprised of our CEO and our Chief Human Resources Officer. The equity award granting authority delegated to the Equity Grant Committee applies tonon-executive employees and covers awards of stock options and RSUs within specific ranges set forth in the policy, which will be updated annually by the Compensation Committee. The Amended and Restated Equity Award Grant Policy sets forth that equity awards are generally granted on the following regularly scheduled basis: Equity awards granted in connection with the hiring of a new employee or the engagement of a new consultant are effective on the first trading day of the month following the later of the date on which such individual’s employment or consulting term begins or the date on which such award is approved by the Board, the Compensation Committee or the Equity Grant Committee. Equity awards granted in connection with the promotion of an existing employee are effective on the first trading day of the month following the later of the date on which such individual’s promotion occurs or the date on which such award is approved by the Board, the Compensation Committee or the Equity Grant Committee; provided, that in the case of any promotion effective on the first trading day of a particular month, the award will be effective on the effective date of such promotion so long as the Board, the Compensation Committee or the Equity Grant Committee approves the award on or before such date. Equity awards granted to existing employees (other than in connection with a promotion) will generally be granted, if at all, on an annual basis effective on the first trading day of the month following the later of the date on which we complete the focal review process with respect to such individual or the date on which such award is approved by the Board, the Compensation Committee or the Equity Grant Committee. Our Board of Directors and the Compensation Committee retain the discretion to grant equity awards at other times to the extent appropriate in light of the circumstances of such awards. In addition, the policy sets forth the manner in which our equity awards will be priced. If equity awards are denominated in dollars, the number of shares subject to the award will be determined by dividing the dollar value by the closing market price on the NASDAQ Global Market of a share of our common stock on the effective date of grant or if no closing price is reported for such date, the closing price on the last date preceding such date for which a closing price is reported. The exercise price of all stock options will be at least equal to the closing market price on the NASDAQ Global Market of a share of our common stock on the effective date of grant or if no closing price is reported for such date, the closing price on the last date preceding such date for which a closing price is reported. If the amount of a stock option award is to be determined by reference to a fair value calculated under Financial Accounting Standards Board Accounting Standard Codification Topic 718,Stock Compensation (formerly FASB Statement No. 123R), or FASB ASC Topic 718, then the number of shares to be subject to such stock option will be determined based on such fair value, and the exercise price determined in accordance with the preceding sentence and the approved valuation assumptions, subject to any other limits on the number of shares that may be subject to such stock option. Policy Prohibiting Hedging and Pledging Our Insider Trading Policy prohibits our executive officers, thenon-employee members of our Board of Directors and certain other employees from engaging in the following transactions: selling any of our securities that they do not own at the time of the sale (a “short sale”); buying or selling puts, calls, other derivative securities of the Company or any derivative securities that provide the economic equivalent of ownership of any of our securities or an opportunity, direct or indirect, to profit from any change in the value of our securities or engaging in any other hedging transaction with respect to our securities at any time without the prior approval of the Audit Committee of our Board of Directors, or Audit Committee; using our securities as collateral in a margin account; and pledging our securities as collateral for a loan (or modifying an existing pledge) unless the pledge has been approved by the Audit Committee. As of the date of this Proxy Statement, none of our NEOs had previously sought or obtained approval from the Audit Committee to engage in any hedging or pledging transaction involving our securities. Stock Ownership Policy In March 2020, we adopted a stock ownership policy for our senior executive officers (i.e., our CEO and each member of our senior management team, which includes our NEOs), which requires (i) our CEO to acquire and hold a number of shares of our common stock equal in value to at least six times his or her annual base salary and (ii) each senior management team member to acquire and hold a number of shares of our common stock equal in value to at least two times his or her applicable annual base salary, in each case until such executive’s service as our CEO or senior management team member, respectively, ceases. We only count directly and beneficially owned shares, including shares purchased through our ESPP or 401(k) plan, and 50% of shares underlying vested and unexercisedin-the-money stock options. Each executive has until the later of the Initial Determination Date or the April 1st in the year that is the fifth anniversary of his or her initial appointment in the capacity of an executive to attain the required ownership level. Once an executive satisfies his or her stock ownership requirement, the executive must continue to satisfy such stock ownership requirement as assessed on each Determination Date. If an executive fails to satisfy such stock ownership requirement as of any Determination Date (including the Initial Determination Date, as applicable), then such executive shall be required to come into compliance with his or her applicable stock ownership requirement within two years following the Determination Date on which he or she failed to satisfy such stock ownership requirement. Tax and Accounting Considerations Deductibility of Executive Compensation Generally, Section 162(m) of the Code, or Section 162(m), disallows a federal income tax deduction for public corporations of remuneration in excess of $1 million paid in any fiscal year to certain specified executive officers. For taxable years beginning before January 1, 2018, (i) these executive officers consisted of a public corporation’s chief executive officer and up to three other executive officers (other than the chief financial officer) whose compensation is required to be disclosed to stockholders under the Securities Exchange Act of 1934 because they are our most highly-compensated executive officers and (ii) qualifying “performance-based compensation” was not subject to Dr. Love’s executionthis deduction limit if specified requirements are met. Pursuant to the Tax Cuts andnon-revocation Jobs Act of 2017, which was signed into law on December 22, 2017, or Tax Act, for taxable years beginning after December 31, 2017, the remuneration of a separation agreementpublic corporation’s chief financial officer is also subject to the deduction limit. In addition, subject to certain transition rules (which apply to remuneration provided pursuant to written binding contracts which were in effect on November 2, 2017, and release, resignationwhich are not subsequently modified in any material respect), for taxable years beginning after December 31, 2017, the exemption from the deduction limit for “performance-based compensation” is no longer available. Consequently, for fiscal years beginning after December 31, 2017, all positions with usremuneration in excess of $1 million paid to a specified executive will not be deductible. In designing our executive compensation program and compliance with our instructions regarding Company property. Underdetermining the CEO Employment Agreement, the term “cause” is generally defined as follows:
“cause” means (i) dishonest statements or acts with respect to us or anycompensation of our affiliates,executive officers, including our NEOs, the Compensation Committee considers a variety of factors, including the potential impact of the Section 162(m) deduction limit. However, the Compensation Committee will not necessarily limit executive compensation to that which is or any current or prospective customers, suppliers vendors or other third partiesmay be deductible under Section 162(m). The Compensation Committee will consider various alternatives to preserving the deductibility of compensation payments and benefits to the extent consistent with whichits compensation goals.
To maintain flexibility to compensate our executive officers in a manner designed to promote our short- and long-term corporate goals, the Compensation Committee has not adopted a policy that all compensation must be deductible. The Compensation Committee believes that our stockholders’ interests are best served if its discretion and flexibility in awarding compensation is not restricted in order to allow such entity does business; (ii) commissioncompensation to be consistent with the goals of (A) a felony or (B) any misdemeanor involving moral turpitude, deceit, dishonesty or fraud; (iii) failureour executive compensation program, even though some compensation awards may result innon-deductible compensation expense. Accounting for Stock-Based Compensation We follow FASB ASC Topic 718 for our stock-based compensation awards. FASB ASC Topic 718 requires us to perform assigned duties and responsibilitiesmeasure the compensation expense for all share-based payment awards made to our reasonable satisfaction, which failure continues, in our reasonable judgment, after written notice by us; (iv) gross negligence, willful misconduct or insubordination with respect to us or anyemployees andnon-employee members of our affiliates;Board of Directors, including stock options to purchase shares of our common stock and other stock awards, based on the grant date “fair value” of these awards. This calculation is performed for accounting purposes and reported in the executive compensation tables required by the federal securities laws, even though the recipient of the awards may never realize any value from their awards. Taxation of “Parachute” Payments Sections 280G and 4999 of the Code provide that executive officers and directors who hold significant equity interests and certain other service providers may be subject to significant additional taxes if they receive payments or (v) material violation of any provision of any agreement(s) between Dr. Love and us relating to noncompetition, nonsolicitation, nondisclosure and/or assignment of inventions. Dr. Love is also eligible to receive certain post-termination compensation and benefits in connection with a change in control of the company that exceeds certain prescribed limits, and that the company (or a successor) may forfeit a deduction on the amounts subject to this additional tax. We have not agreed to provide any executive officer, including any NEO, with a“gross-up” or other reimbursement payment for any tax liability that the executive officer might owe as a result of the application of Sections 280G or 4999 of the Code.
Section 409A of the Internal Revenue Code Section 409A of the Code imposes additional significant taxes in accordancethe event that an executive officer, director or service provider receives “deferred compensation” that does not satisfy the requirements of Section 409A of the Code. Although we do not maintain a traditional nonqualified deferred compensation plan, Section 409A of the Code does apply to certain severance arrangements, bonus arrangements and equity awards. We structure all our severance arrangements, bonus arrangements and equity awards in a manner to either avoid the application of Section 409A or, to the extent doing so is not possible, to comply with the Policy described above.applicable requirements of Section 409A of the Code. Jeffrey FarrowCompensation Risk Assessment
We entered into an employment offer letter agreement with Mr. Farrow, pursuantstructure our pay to which he assumedconsist of both fixed and variable compensation to motivate our employees, including our NEOs, to produce superior short- and long-term results that are in the rolebest interests of Chief Financial Officer in April 2016. The agreement entitles Mr. Farrowour company and stockholders to an annual base salary that is currently set at $415,000, subject to adjustment pursuant toattain our employee compensation policies in effect from time to time. Pursuant to the termsultimate objective of his agreementincreasing stockholder value. In addition, we have established, and the Incentive Plan, Mr. Farrow is eligible to receive annual incentive compensation at a target percentage (currently 40%) of his then-current annual base salary, as determined by the Compensation Committee endorses, several controls to address and mitigate compensation-related risk, such as maintaining an anti-hedging and anti-pledging policy and stock ownership guidelines for our executive officers (including our NEOs) and directors. The Compensation Committee, in consultation with its compensation consultant, Compensia, evaluates whether our policies and practices create excessive risk in our compensation programs. In 2019, this risk assessment included, among other things, a review of our Boardcash and equity incentive-based compensation plans to ensure that they are aligned with our corporate performance goals and overall target total direct compensation to ensure an appropriate balance between fixed and variable pay components. Based on this assessment, the Compensation Committee concluded that our compensation policies and practices are not reasonably likely to have a material adverse effect on our company. The Compensation Committee intends to continue to evaluate on an annual basis the potential risks associated with our compensation policies and practices, and has engaged Compensia to conduct an updated assessment of Directors. Pursuantour compensation policies and practices during 2020. As a result of the approval and launch of our first commercial product, Oxbryta, in late 2019, we expect this evaluation will include the potential risks associated with field-based incentive compensation and commercial-related goals and targets. 2019 Summary Compensation Table The following table sets forth information regarding total compensation awarded to, earned by and paid to each of our NEOs during the fiscal years ended December 31, 2019, 2018 and 2017 to the extent he was an NEO in such year. | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Name and Principal Position | | Year | | | Salary
($) | | | Bonus
($) | | | Stock Awards
($)(1) | | | Option
Awards
($)(2) | | | Non-Equity
Incentive
Plan
Compensation
($)(3) | | | All Other
Compensation
($) | | | Total
($) | | Ted Love, M.D. | | | 2019 | | | | 596,876 | (4) | | | — | | | | 4,359,600 | | | | 4,595,717 | | | | 540,000 | | | | 5,000 | (5) | | | 10,097,193 | | President, Chief Executive Officer and Director | | | 2018 2017 | | | | 568,750 520,000 | | | | — — | | | | 4,648,800 5,559,125 | | | | 4,623,690 1,557,373 | | | | 414,000 380,000 | | | | 5,000 2,500 | | | | 10,260,240 8,018,998 | | Jeffrey Farrow | | | 2019 | | | | 440,625 | (6) | | | — | | | | 1,211,000 | | | | 1,267,784 | | | | 244,000 | | | | 5,000 | (5) | | | 3,168,409 | | Chief Financial Officer | |
| 2018
2017 |
| |
| 425,938
412,945 |
| |
| —
— |
| |
| 1,370,800
629,030 |
| |
| 1,326,469
397,398 |
| |
| 201,000
200,000 |
| |
| 5,000
11,296 |
| |
| 3,329,207
1,650,669 |
| Brian Cathers | | | 2019 | | | | 319,445 | (7) | | | — | | | | 1,604,400 | | | | 1,571,112 | | | | 172,000 | (7) | | | 100,236 | (5) | | | 3,767,193 | | Chief Scientific Officer | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | David Johnson(8) | | | 2019 | | | | 453,125 | (9) | | | — | | | | 1,211,000 | | | | 1,267,784 | | | | 250,000 | | | | 5,000 | (5) | | | 3,186,909 | | Chief Commercial Officer | | | 2018 | | | | 355,000 | | | | 75,000 | | | | 1,891,750 | | | | 1,898,534 | | | | 169,000 | | | | 5,000 | | | | 4,394,284 | | Joshua Lehrer-Graiwer(10) | | | 2019 | | | | 430,625 | (11) | | | — | | | | 1,208,825 | | | | 1,258,161 | | | | 253,000 | | | | 5,000 | (5) | | | 3,155,611 | | Chief Medical Officer | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| (1) | In accordance with SEC rules, this column reflects the aggregate grant date fair value of the restricted stock and/or RSUs granted during 2017, 2018 and 2019, as applicable, computed in accordance with FASB ASC Topic 718. Assumptions used in the calculation of these amounts are included in Note 10 to our financial statements included in our Annual Report on Form10-K for the fiscal year ended December 31, 2019. These amounts do not reflect the actual economic value that may be realized by the NEOs upon the vesting or settlement of the restricted stock or RSUs, as applicable, or the sale of the common stock underlying such awards. |
| (2) | In accordance with SEC rules, this column reflects the aggregate grant date fair value of the stock option awards granted during 2017, 2018 and 2019, as applicable, computed in accordance with FASB ASC Topic 718. Assumptions used in the calculation of these amounts are included in Note 10 to our financial statements included in our Annual Report on Form10-K for the fiscal year ended December 31, 2019. These amounts do not reflect the actual economic value that may be realized by the NEOs upon the exercise of the stock options or the sale of the common stock underlying such stock options. |
| (3) | The amounts reported reflect the annual cash incentive compensation earned by the NEOs under our Cash Incentive Plan, based on our achievement of certain corporate performance goals and each NEO’s (except for the CEO) achievement of his individual performance goals. |
| (4) | From January 1, 2019, Dr. Love’s annual base salary was $575,000, which increased to $600,000, effective February 16, 2019. |
| (5) | The amounts reported consist of employer matching contributions under our 401(k) of $5,000 each for each NEO other than for Dr. Cathers. The amount reported for Dr. Cathers consists of employer matching contributions under our 401(k) of $236 and monthly stipends to cover his travel expenses in connection with his employment for an aggregate of $100,000. |
| (6) | From January 1, 2019, Mr. Farrow’s annual base salary was $427,500, which increased to $442,500, effective February 16, 2019. |
| (7) | Dr. Cathers commenced employment with us on February 25, 2019, and his annual base salary and annual cash incentive compensation amounts were prorated accordingly. |
| (8) | Mr. Johnson commenced employment with us on March 12, 2018 and was not an NEO for 2017. |
| (9) | From January 1, 2019, Mr. Johnson’s annual base salary was $440,000, which increased to $455,000, effective February 16, 2019. |
| (10) | Dr. Lehrer-Graiwer was not an NEO for 2018 or 2017. |
| (11) | From January 1, 2019, Dr. Lehrer-Graiwer’s annual base salary was $400,000, which increased to $425,000, effective February 16, 2019, and $460,000, effective October 1, 2019. |
Grants of Plan-Based Awards for Fiscal Year 2019 The following table sets forth the individual awards made to each of our NEOs during 2019. For a description of the types of awards indicated below, please see our “Compensation Discussion and Analysis” above. | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Estimated
Future
Payouts
Under
Non-
Equity
Incentive
Plan
Awards(1) | | | All Other
Stock
Awards:
Number of
Shares of
Stock or
Units
(#)(2) | | | All Other
Option
Awards:
Number
of
Securities
Underlying
Options
(#)(3) | | | Exercise
or Base
Price of
Option
Awards
($/sh.) | | | Grant
Date Fair
Market
Value of
Awards
($)(4) | | Name | | | | Grant
Date | | | Target
($) | | Ted Love, M.D. | | Time-Based RSUs | | | 2/1/2019 | | | | — | | | | 90,000 | | | | — | | | | — | | | | 4,359,600 | | | | Time-Based Stock
Options | | | 2/1/2019 | | | | — | | | | — | | | | 145,000 | | | | 48.44 | | | | 4,595,717 | | | | Annual Bonus
Opportunity | | | | | | | 360,000 | | | | — | | | | — | | | | — | | | | — | | | | | | | | | | Jeffrey Farrow | | Time-Based RSUs | | | 2/1/2019 | | | | — | | | | 25,000 | | | | — | | | | — | | | | 1,211,000 | | | | Time-Based Stock
Options | | | 2/1/2019 | | | | — | | | | — | | | | 40,000 | | | | 48.44 | | | | 1,267,784 | | | | Annual Bonus
Opportunity | | | | | | | 177,000 | | | | — | | | | — | | | | — | | | | — | | | | | | | | | | Brian Cathers | | Time-Based RSUs | | | 3/1/2019 | | | | — | | | | 30,000 | | | | — | | | | — | | | | 1,604,400 | | | | Time-Based Stock
Options | | | 3/1/2019 | | | | — | | | | — | | | | 45,000 | | | | 53.48 | | | | 1,571,112 | | | | Annual Bonus
Opportunity | | | | | | | 150,000 | (5) | | | — | | | | — | | | | — | | | | — | | | | | | | | | | David Johnson | | Time-Based RSUs | | | 2/1/2019 | | | | — | | | | 25,000 | | | | — | | | | — | | | | 1,211,000 | | | | Time-Based Stock
Options | | | 2/1/2019 | | | | — | | | | — | | | | 40,000 | | | | 48.44 | | | | 1,267,784 | | | | Annual Bonus
Opportunity | | | | | | | 182,000 | | | | — | | | | — | | | | — | | | | — | | | | | | | | | | Joshua Lehrer-Graiwer | | Time-Based RSUs | | | 2/1/2019 | | | | — | | | | 23,500 | | | | — | | | | — | | | | 1,138,340 | | | | | | | 10/1/2019 | | | | — | | | | 1,500 | | | | — | | | | — | | | | 70,485 | | | | Time-Based Stock
Options | | | 2/1/2019 | | | | — | | | | — | | | | 35,000 | | | | 48.44 | | | | 1,109,311 | | | | | | | 10/1/2019 | | | | — | | | | — | | | | 5,000 | | | | 46.99 | | | | 148,850 | | | | Annual Bonus
Opportunity | | | | | | | 184,000 | | | | — | | | | — | | | | — | | | | — | |
| (1) | The amounts shown reflect the target cash incentive compensation for our NEOs, which are disclosed in the “2019 Target Annual Bonus” section of the Compensation Discussion and Analysis. The actual amounts paid for 2019 are disclosed in the“Non-Equity Incentive Plan Compensation” column of the Summary Compensation Table. There were no threshold or maximum payout levels for the cash incentive compensation. |
| (2) | The amounts shown represent time-based RSUs granted pursuant to our 2015 Plan or 2017 Inducement Plan, which amounts will be payable in shares of our common stock, if the service-based conditions for such time-based RSUs are met. Except for Dr. Cathers’ time-based RSUs, the time-based RSUs vest semi-annually over four years, subject to the NEO’s continuous service relationship with us through each applicable vesting date. Dr. Cathers’ time-based RSUs vest over a four-year period, with 1/4th of the underlying shares vesting on the first anniversary of the vesting commencement date of March 1, 2019, and thereafter 1/6th of the remaining underlying shares vest on a semi-annual basis (every six months) so that all of the underlying shares will be vested on the date four years after the vesting commencement date, subject to Dr. Cathers’ continuous service relationship with us through each applicable vesting date. |
| (3) | The amounts shown represent time-based stock options granted pursuant to our 2015 Plan or 2017 Inducement Plan. Except for Dr. Cathers’ time-based stock options, the time-based stock options vest quarterly over four years, subject to the NEO’s continuous service relationship with us through each applicable vesting date. Dr. Cathers’ time-based options vest, and become exercisable, over a four-year period, with 1/4th of the underlying shares vesting on the first anniversary of the vesting commencement date of February 25, 2019, and thereafter, 1/12th of the remaining underlying shares vest on a quarterly basis so that all of the underlying shares will be vested on the date four years after the vesting commencement date, subject to Dr. Cathers continuous service relationship with us through each applicable vesting date. |
| (4) | In accordance with SEC rules, this column reflects the aggregate grant date fair value of the equity awards granted during 2019, computed in accordance with FASB ASC Topic 718. Assumptions used in the calculation of these amounts are included in Note 10 to our financial statements included in our Annual Report on Form10-K for the fiscal year ended December 31, 2019. These amounts do not reflect the actual economic value that may be realized by the NEOs upon the vesting or settlement of RSUs or the exercise of the stock options, as applicable, or the sale of the common stock underlying such awards. |
| (5) | Dr. Cathers commenced employment with us on February 25, 2019. The amount reflects Dr. Cathers’ annualized target cash incentive compensation. |
2019 Outstanding Equity Awards at Fiscal Year End The following table sets forth certain information with respect to outstanding equity awards held by each of our NEOs as of December 31, 2019. | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Option Awards(1) | | | Stock Awards(1) | | Name | | Number of
Securities
Underlying
Unexercised
Options (#)
Exercisable | | | Number of
Securities
Underlying
Unexercised
Options (#)
Unexercisable | | | Equity
Incentive
Plan
Awards:
Number of
Securities
Underlying
Unexercised
Unearned
Options
(#) | | | Option
Exercise
Price
($) | | | Option
Expiration
Date | | | Number
of
shares
or units
of stock
that
have
not
vested
(#)(1) | | | Market
value of
shares or
units of
stock that
have
not
vested
($)(2) | | | Equity
Incentive
Plan
Awards:
Number of
unearned
shares, units
or
other rights
that have
not vested
(#) | | | Equity
Incentive
Plan
Awards:
Market
value or
payout
value of
unearned
shares, units
or other
rights
that have
not
vested
($)(2) | | | (a) | | (b) | | | (c) | | | (d) | | | (e) | | | (f) | | | (g) | | | (h) | | | (i) | | | (j) | | | | | | | | | | | | Ted Love, M.D. | | | — | | | | — | | | | — | | | | — | | | | — | | | | 36,188 | (3) | | | 2,876,584 | | | | — | | | | — | | | | | | | | | | | | | | | 87,493 | (4) | | | 45,313 | (4) | | | — | | | | 16.40 | | | | 1/17/2027 | | | | — | | | | — | | | | — | | | | — | | | | | | | | | | | | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 48,750 | (5) | | | 3,875,138 | | | | — | | | | — | | | | | | | | | | | | | | | 53,375 | (6) | | | 68,625 | (6) | | | — | | | | 59.60 | | | | 2/1/2028 | | | | — | | | | — | | | | — | | | | — | | | | | | | | | | | | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 78,750 | (7) | | | 6,259,838 | | | | — | | | | — | | | | | | | | | | | | | | | 27,187 | (8) | | | 117,813 | (8) | | | — | | | | 48.44 | | | | 2/1/2029 | | | | — | | | | — | | | | — | | | | — | | | | | | | | | | | | Jeffrey Farrow | | | 105,000 | (9) | | | 15,000 | (9) | | | — | | | | 14.96 | | | | 2/24/2026 | | | | — | | | | — | | | | — | | | | — | | | | | | | | | | | | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 9,188 | (3) | | | 730,354 | | | | — | | | | — | | | | | | | | | | | | | | | 22,389 | (4) | | | 11,563 | (4) | | | — | | | | 16.40 | | | | 1/17/2027 | | | | — | | | | — | | | | — | | | | — | | | | | | | | | | | | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 14,375 | (5) | | | 1,142,669 | | | | — | | | | — | | | | | | | | | | | | | | | 15,312 | (6) | | | 19,688 | (6) | | | — | | | | 59.60 | | | | 2/1/2028 | | | | — | | | | — | | | | — | | | | — | | | | | | | | | | | | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 21,875 | (7) | | | 1,738,844 | | | | — | | | | — | | | | | | | | | | | | | | | 7,500 | (8) | | | 32,500 | (8) | | | — | | | | 48.44 | | | | 2/1/2029 | | | | — | | | | — | | | | — | | | | — | | | | | | | | | | | | Brian Cathers | | | — | | | | — | | | | — | | | | — | | | | — | | | | 30,000 | (10) | | | 2,384,700 | | | | — | | | | — | | | | | | | | | | | | | | | — | (11) | | | 45,000 | (11) | | | — | | | | 53.48 | | | | 3/1/2029 | | | | — | | | | — | | | | — | | | | — | | | | | | | | | | | | David Johnson | | | — | | | | — | | | | — | | | | — | | | | — | | | | 21,875 | (12) | | | 1,738,844 | | | | — | | | | — | | | | | | | | | | | | | | | 24,062 | (13) | | | 30,938 | (13) | | | — | | | | 54.05 | | | | 3/12/2028 | | | | — | | | | — | | | | — | | | | — | | | | | | | | | | | | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 21,875 | (7) | | | 1,738,844 | | | | — | | | | — | | | | | | | | | | | | | | | 7,500 | (8) | | | 32,500 | (8) | | | — | | | | 48.44 | | | | 2/1/2029 | | | | — | | | | — | | | | — | | | | — | | | | | | | | | | | | Joshua Lehrer-Graiwer | | | — | (14) | | | 625 | (14) | | | — | | | | 12.95 | | | | 2/9/2026 | | | | — | | | | — | | | | — | | | | — | | | | | | | | | | | | | | | — | (15) | | | 2,813 | (15) | | | — | | | | 19.89 | | | | 9/8/2026 | | | | — | | | | — | | | | — | | | | — | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 5,250 | (16) | | | 417,323 | | | | | | | | | | | | | | | | | | | | | | | — | (17) | | | 6,563 | (17) | | | | | | | 26.90 | | | | 2/17/2027 | | | | — | | | | — | | | | — | | | | — | | | | | | | | | | | | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 4,150 | (18) | | | 329,884 | | | | — | | | | — | | | | | | | | | | | | | | | — | (19) | | | 5,469 | (19) | | | | | | | 27.90 | | | | 7/26/2027 | | | | — | | | | — | | | | — | | | | — | | | | | | | | | | | | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 10,625 | (5) | | | 844,581 | | | | — | | | | — | | | | | | | | | | | | | | | — | (6) | | | 14,907 | (6) | | | — | | | | 59.60 | | | | 2/1/2028 | | | | — | | | | — | | | | — | | | | — | | | | | | | | | | | | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 20,563 | (7) | | | 1,634,553 | | | | — | | | | — | | | | | | | | | | | | | | | — | (8) | | | 28,438 | (8) | | | — | | | | 48.44 | | | | 2/1/2029 | | | | — | | | | — | | | | — | | | | — | | | | | | | | | | | | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 1,500 | (20) | | | 119,235 | | | | — | | | | — | | | | | | | | | | | | | | | — | (21) | | | 5,000 | (21) | | | | | | | 46.99 | | | | 10/1/2029 | | | | — | | | | — | | | | — | | | | — | |
| (1) | All of the outstanding equity awards held by our NEOs will become fully vested and exercisable ornon-forfeitable, as applicable, if the NEO is terminated without Cause or resigns for Good Reason, in either case, within 12 months following a Sale Event. The vesting acceleration of the outstanding equity awards held by our NEOs is described in greater detail in “Employment Arrangements with Our Named Executive Officers—Change in Control Policy”. |
| (2) | Computed in accordance with SEC rules as the number of unvested shares or units multiplied by the closing market price of a share of our common stock on December 31, 2019, which was $79.49. The actual value (if any) to be realized by the NEO depends on whether the shares or units vest and the future performance of our common stock. |
| (3) | 1/8th of the RSUs vest on a semi-annual basis from the vesting commencement date of January 17, 2017, such that all of the RSUs will be fully vested on January 17, 2021, provided the NEO remains in continuous service through each such vesting date. |
| (4) | 1/16th of the shares subject to the stock option vest on a quarterly basis from the vesting commencement date of January 17, 2017, such that all of the shares will be fully vested on January 17, 2021, provided the NEO remains in continuous service through each such vesting date. |
| (5) | 1/8th of the RSUs vest on a semi-annual basis from the vesting commencement date February 1, 2018, such that all of the RSUs will be fully vested on February 1, 2022, provided the NEO remains in continuous service through each such vesting date. |
| (6) | 1/16th of the shares subject to the stock option vest on a quarterly basis from the vesting commencement date of February 1, 2018, such that all of the shares will be fully vested on February 1, 2022, provided the NEO remains in continuous service through each such vesting date. |
| (7) | 1/8th of the RSUs vest on a semi-annual basis from the vesting commencement date February 1, 2019, such that all of the RSUs will be fully vested on February 1, 2023, provided the NEO remains in continuous service through each such vesting date. |
| (8) | 1/16th of the shares subject to the stock option vest on a quarterly basis from the vesting commencement date of February 1, 2019, such that all of the shares will be fully vested on February 1, 2023, provided the NEO remains in continuous service through each such vesting date. |
| (9) | 25% of the shares subject to the stock option vested on April 4, 2017, and the remaining shares vest quarterly over the following three years thereafter, such that all of the shares will be fully vested on April 4, 2020, subject to the NEO’s continuous service through each such vesting date. |
| (10) | 25% of the RSUs vested on March 1, 2020, and 1/8th of the RSUs vest on a semi-annual basis from such date, such that all of the RSUs will be fully vested on March 1, 2023, provided the NEO remains in continuous service through each such vesting date. |
| (11) | 25% of the shares subject to the stock option vested on the first anniversary of the vesting commencement date of February 25, 2019, and the remaining shares vest quarterly over the following three years thereafter, such that all of the shares will be fully vested on February 25, 2023, subject to the NEO’s continuous service through each such vesting date. |
| (12) | 25% of the RSUs vested on April 1, 2019 and 1/8th of the RSUs vest on a semi-annual basis from such date, such that all of the RSUs will be fully vested on April 1, 2022, provided the NEO remains in continuous service through each such vesting date. |
| (13) | 25% of the shares subject to the stock option vested on March 12, 2019 and 1/16th of the shares subject to the stock option vest on a quarterly basis from such date, such that all of the shares will be fully vested on March 12, 2022, provided the NEO remains in continuous service through each such vesting date. |
| (14) | 1/16th of the shares subject to the stock option vest on a quarterly basis from the vesting commencement date of February 9, 2016, such that all of the shares vested on February 9, 2020. |
| (15) | 1/16th of the shares subject to the stock option vest on a quarterly basis from the vesting commencement date of September 8, 2016, such that all of the shares will be fully vested on September 8, 2020, provided the NEO remains in continuous service through each such vesting date. |
| (16) | 1/8th of the RSUs vest on a semi-annual basis from the vesting commencement date of March 1, 2017, such that all of the RSUs will be fully vested on March 1, 2021, provided the NEO remains in continuous service through each such vesting date. |
| (17) | 1/16th of the shares subject to the stock option vest on a quarterly basis from the vesting commencement date of February 17, 2017, such that all of the shares will be fully vested on February 17, 2021, provided the NEO remains in continuous service through each such vesting date. |
| (18) | 1/8th of the RSUs vest on a semi-annual basis from the vesting commencement date of August 1, 2017, such that all of the RSUs will be fully vested on August 1, 2021, provided the NEO remains in continuous service through each such vesting date. |
| (19) | 1/16th of the shares subject to the stock option vest on a quarterly basis from the vesting commencement date of July 26, 2017, such that all of the shares will be fully vested on July 26, 2021, provided the NEO remains in continuous service through each such vesting date. |
| (20) | 1/8th of the RSUs vest on a semi-annual basis from the vesting commencement date of October 1, 2019, such that all of the RSUs will be fully vested on October 1, 2023, provided the NEO remains in continuous service through each such vesting date. |
| (21) | 1/16th of the shares subject to the stock option vest on a quarterly basis from the vesting commencement date of October 1, 2019, such that all of the shares will be fully vested on October 1, 2023, provided the NEO remains in continuous service through each such vesting date. |
Option Exercises and Stock Vested in Fiscal Year 2019 The following table sets forth the number of shares acquired and the value realized upon exercises of stock options and vesting of RSUs during the fiscal year ended December 31, 2019 by each of our NEOs. | | | | | | | | | | | | | | | | | | | | Option Awards | | | Stock Awards | | Name | | Number of Shares
Acquired on
Exercise
(#) | | | Value Realized
on Exercise
($)(1) | | | Number of Shares
Acquired on
Vesting
(#) | | | Value Realized on
Vesting
($)(2) | | | | | | | Ted Love, M.D. | | | — | | | | — | | | | 202,553 | | | | 12,889,776 | | | | | | | Jeffrey Farrow | | | — | | | | — | | | | 22,000 | | | | 1,280,670 | | | | | | | Brian Cathers | | | — | | | | — | | | | — | | | | — | | | | | | | David Johnson | | | — | | | | — | | | | 16,250 | | | | 837,988 | | | | | | | Joshua Lehrer-Graiwer | | | 50,354 | | | | 1,204,657 | | | | 18,362 | | | | 1,059,283 | |
| (1) | The value realized upon the exercise of stock options is calculated by (a) subtracting the stock option exercise price from the market price on the date of exercise to get the realized value per share, and (b) multiplying the realized value per share by the number of shares underlying the stock options exercised. |
| (2) | The value realized upon vesting of restricted stock and RSUs is calculated by multiplying the number of shares of restricted stock and RSUs vested by the market price on the vest date. |
Potential Payments on Termination or Change in Control Our Amended and Restated Severance and Change in Control Policy provides for certain payments and benefits to each of our NEOs if the NEO is terminated by us or our acquirer or successor without Cause or resigns for Good Reason (as such terms are defined in such policy), both during and outside of histhe Change in Control Period (assuming that all outstanding equity awards were assumed, continued or substituted by an acquirer or successor in such change in control), subject to the NEO’s execution andnon-revocation of a severance agreement, Mr. Farrow was issued an optionincluding a general release of claims. The table below quantifies the potential payments and benefits that would have become due to purchase 120,000 sharesour NEOs assuming that Amended and Restated Severance and Change in Control Policy had been in effect as of December 31, 2019 and that one of the triggering events above occurred as of December 31, 2019. The market price of a share of our common stock on December 31, 2019 was $79.49. Prior to the adoption of the Amended and Restated Severance and Change in Control Policy in January 2020, none of our NEOs except for our CEO was entitled to termination benefits outside of the Change in Control Period. | | | | | | | | | Name | | Qualifying
Termination
Not in
Connection
with a Change
in Control
($) | | | Qualifying
Termination
in
Connection
with a Change
in Control
($) | | Ted Love, M.D. | | | | | | | | | Cash Severance Payment | | | 600,000 | (1) | | | 900,000 | (2) | Cash Incentive Bonus Payment | | | 360,000 | (3) | | | 540,000 | (4) | COBRA Premiums | | | 16,154 | (5) | | | 24,231 | (6) | Accelerated Equity Vesting (Time-Based) | | | — | | | | 17,235,308 | (7) | Jeffrey Farrow | | | | | | | | | Cash Severance Payment | | | 442,500 | (1) | | | 442,500 | (1) | Cash Incentive Bonus Payment | | | — | | | | 177,000 | (3) | COBRA Premiums | | | 11,285 | (5) | | | 11,285 | (5) | Accelerated Equity Vesting (Time-Based) | | | — | | | | 6,710,046 | (7) | Brian Cathers | | | | | | | | | Cash Severance Payment | | | 375,000 | (1) | | | 375,000 | (1) | Cash Incentive Bonus Payment | | | — | | | | 150,000 | (3) | COBRA Premiums | | | 11,719 | (5) | | | 11,719 | (5) | Accelerated Equity Vesting (Time-Based) | | | — | | | | 3,555,150 | (7) | David Johnson | | | | | | | | | Cash Severance Payment | | | 455,000 | (1) | | | 455,000 | (1) | Cash Incentive Bonus Payment | | | — | | | | 182,000 | (3) | COBRA Premiums | | | 11,719 | (5) | | | 11,719 | (5) | Accelerated Equity Vesting (Time-Based) | | | — | | | | 5,273,875 | (7) |
| (1) | Represents 12 months of the NEO’s base salary. |
| (2) | Represents 18 months of Dr. Love’s base salary. |
| (3) | Represents 100% of the NEO’s target annual bonus opportunity. The table does not include the prorated annual bonus opportunity that the NEO would be entitled to since, on December 31, 2019, the full annual bonus, which is shown in the “Summary Compensation Table,” was earned. |
| (4) | Represents 150% of Dr. Love’s target annual bonus opportunity. The table does not include the prorated annual bonus opportunity that Dr. Love would be entitled to since, on December 31, 2019, the full annual bonus, which is shown in the “Summary Compensation Table,” was earned. |
| (5) | Represents 12 months of our contribution towards health insurance, based on our actual costs to provide health insurance to the NEO as of the date of termination. |
| (6) | Represents 18 months of our contribution towards health insurance, based on our actual costs to provide health insurance to the NEO as of the date of termination. |
| (7) | Represents the value of acceleration of vesting of 100% of the NEO’s unvested and outstanding equity awards, based on the market price of a share of our common stock on December 31, 2019, which was $79.49. For stock options with a per share exercise price greater than $79.49, no amount was included with respect to such stock options. |
In February 24, 2016. Mr. Farrow is also eligible to receive certain post-termination compensation and benefits in2020, Dr. Lehrer-Graiwer resigned from his employment with us, effective April 17, 2020. In connection with a change in control in accordance with the Policy described above.
Tricia Suvari
We entered into an employment offer letter agreement with Ms. Suvari, pursuant to which she assumed the roleDr. Lehrer-Graiwer’s resignation, he did not receive any severance benefits or payments or acceleration of Chief Legal Officer in October 2016. The agreement entitles Ms. Suvari to an annual base salary that is currently set at $365,000, subject to adjustment pursuant to our employee compensation policies in effect from time to time. Pursuant to the terms of her agreement and the Incentive Plan, Ms. Suvari is eligible to receive annual incentive compensation at a target percentage (currently 40%) of her then-current annual base salary, as determined by the Compensation Committee of our Board of Directors. Pursuant to the terms of her agreement, Ms. Suvari was issued an option to purchase 100,000 shares of our common stock on October 11, 2016.
Ms. Suvari is also eligible to receive certain post-termination compensation and benefits in connection with a change in control in accordance with the Policy described above.
Termination Letter Agreement with Eleanor L. Ramos, M.D.
We entered into a termination letter agreement and release with Dr. Ramos on November 7, 2016 (the “Termination Agreement”). Pursuant to the terms of the Termination Agreement, the Company agreed, in exchange for a full release of claims from Dr. Ramos, to (i) pay Dr. Ramos an aggregate of $190,000, which represented six additional months of her base salary, less applicable withholding, (ii) reimburse Dr. Ramos for certain benefits continuation through April 30, 2017 and (iii) accelerate the vesting of 54,999 shares subject to Dr. Ramos’ outstanding options.equity awards.
Securities Authorized for Issuance under Equity Compensation Plans Equity Compensation Plans The following table sets forth information as of December 31, 20162019, regarding shares of common stock that may be issued under our equity compensation plans, consisting of our 2012 Stock Option and Grant Plan (or 2012 Plan), our 2015 Plan, our 2017 Inducement Plan and our 2015 Employee Stock Purchase Plan (the “ESPP”).ESPP. | | | | | | | | | | | | | Plan Category | | Number of Securities
to be Issued upon Exercise
of Outstanding Options and
Rights (#)(a) | | | Weighted Average Exercise
Price of Outstanding
Options and Rights (b)(1) | | | Number of Securities
Remaining Available for
Future Issuance under
Equity Compensation Plans
(Excluding Securities
Reflected in Column (a))(c) | | Equity compensation plans approved by security holders(2) | | | 2,769,702 | (3) | | $ | 11.99 | (3) | | | 1,477,271 | (4) | Equity compensation plans not approved by security holders(5) | | | — | | | | — | | | | — | | | | | | | | | | | | | | | Total | | | 2,769,702 | | | $ | 11.99 | | | | 1,477,271 | | | | | | | | | | | | | | |
| | | | | | | | | | | | | Plan Category | | Number of Securities
to be Issued upon Exercise
of Outstanding Options,
Warrants and
Rights (#)(a) | | | Weighted Average Exercise
Price of Outstanding
Options, Warrants and
Rights (b)(1) | | | Number of Securities
Remaining Available for
Future Issuance under
Equity Compensation Plans
(Excluding Securities
Reflected in Column (a))(c) | | | | | | Equity compensation plans approved by security holders(2) | | | 3,963,354 | (3) | | $ | 33.72 | (3) | | | 3,893,761 | (4) | | | | | Equity compensation plans not approved by security holders(5) | | | 1,459,278 | | | $ | 48.18 | | | | 837,550 | | | | | | Total | | | 5,422,632 | | | $ | 36.24 | | | | 4,731,311 | |
| (1) | The weighted average exercise price is calculated based solely on outstanding stock options. |
| (2) | Includes the 2012 Plan, the 2015 Plan and the ESPP, as well as our 2012 Plan.ESPP. Our 2015 Plan provides that the number of shares reserved and available for issuance under the plan will automatically increase each January 1, beginning on January 1, 2016, by 4% of the outstanding number of shares of common stock on the immediately preceding December 31 or such lesser number of shares as determined by the compensation committee of the Company’sour Board of Directors. Our ESPP provides that the number of shares reserved and available for issuance will automatically increase each January 1, from January 1, 2016 until January 1, 2025, by the lesser of (i) 3,000,000 shares of common stock, (ii) 1% of the outstanding number of shares of common stock on the immediately preceding December 31 or (iii) such lesser amountnumber of shares as determined by the compensation committee of the Company’sour Board of Directors. On January 1, 2017,2020, the number of shares available for issuance under our 2015 Plan and our ESPP increased by 1,492,0002,425,694 shares and 186,500100,000 shares, respectively, pursuant to these provisions. These increases are not reflected in the table above. We no longer grant new awards under ourthe 2012 Plan, and any awards previously granted under such plan prior to our initial public offering that are forfeited, canceled, reacquired by us prior to vesting satisfied without the issuance of stock or otherwise terminated (other than by exercise) are added to shares available for issuance under the 2015 Plan. |
| (3) | Does not include purchase rights accruing under the ESPP because the purchase right (and therefore the number of shares to be purchased) will not be determined until the end of the purchase period. |
| (4) | As of December 31, 2016,2019, there were 1,401,1533,641,106 shares of common stock available for issuance under the 2015 Plan and 76,118252,655 shares of common stock available for issuance under the ESPP.ESPP (which number includes shares subject to purchase during the current purchase period, which commenced on February 1, 2020, and the exact number of which will not be known until the end of the purchase period on July 31, 2020. Subject to the number of shares remaining in the share reserve, the maximum number of shares purchasable by any participant on any one purchase date for any purchase period, including the current period, may not exceed 2,500 shares). |
| (5) | Does not include 300,000 shares reserved for issuance pursuant to ourConsists solely of the 2017 Inducement Equity Plan, which was adopted by the Board of Directors in January 2017.Plan. |
As required by Section 953(b) of the Dodd-Frank Act, and Item 402(u) of RegulationS-K, we are disclosing the ratio of the annual total compensation of our CEO to the annual total compensation of our median employee. For 2019: the annual total compensation of our CEO was $10,097,193 (as disclosed in the Summary Compensation Table above); the annual total compensation of our median employee was $321,142; and the ratio of the annual total compensation of our CEO to the annual total compensation of our median employee was 31 to 1. As permitted by the SEC rules, we identified our median employee as of December 31, 2019, by: (i) calculating for each full-time employee, except our CEO, and part-time employee on that date (a) actual annual base salary in 2019 (annualized for new hires who were not employed for the entire year and for permanent employees on an unpaid leave of absence for a portion of the year), (b) actual annual bonus earned in 2019 (annualized for new hires who were not employed for the entire year and for permanent employees on an unpaid leave of absence for a portion of the year), and (c) the grant date fair value of all equity awards granted in 2019; and (ii) ranking this compensation from lowest to highest to identify our median employee. For purposes of this disclosure, earnings of any employee outside the U.S. were converted to U.S. dollars using the monthly average Swiss franc to U.S. dollar exchange rate for each month in 2019, based on exchange rates used by us for various purposes. We used this compensation as our consistently applied compensation measure because we believe it was representative of our employee compensation. After identifying our median employee and ensuring this employee did not have anomalous compensation in 2019, we then calculated the annual total compensation of our median employee using the same methodology we used for our CEO, and our other NEOs, in the Summary Compensation Table above. The pay ratio above is a reasonable estimate calculated in a manner consistent with the SEC rules. The SEC rules provide companies with significant flexibility in identifying the median employee and calculating the pay ratio, including flexibility to adopt a variety of methodologies, to apply certain exclusions and to make reasonable estimates and assumptions that reflect their employee populations and compensation practices. Accordingly, the pay ratio above may not be comparable with the pay ratios of other companies, even companies within our industry. COMPENSATION COMMITTEE REPORT The following Compensation Committee Report is not considered proxy solicitation material and is not deemed filed with the Securities and Exchange Commission. Notwithstanding anything to the contrary set forth in any of our filings made under the Securities Act of 1933 or the Exchange Act that might incorporate our filings under those statutes, the Compensation Committee Report shall not be incorporated by reference into any of our prior filings or into any of our future filings under those statutes. The Compensation Committee of the Board of Directors has reviewed and discussed the Compensation Discussion and Analysis required by Item 402(b) of RegulationS-K with the Company’s management. Based on this review and discussion, the Compensation Committee recommended to the Board of Directors, and the Board of Directors approved, that the Compensation Discussion and Analysis be included in this Proxy Statement for the Annual Meeting and incorporated by reference in the Company’s Annual Report on Form10-K for the fiscal year ended December 31, 2019. COMPENSATION COMMITTEE WENDY YARNO, CHAIRPERSON SCOTT W. MORRISON MARK L. PERRY CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS Other than the compensation agreements and other arrangements with our directors and NEOs described under “Compensation of Executive Officers”above, and the transactions described below, since January 1, 2016,2019, there has not been and there is not currently proposed, any transaction or series of similar transactions to which we were, or will be, a party in which the amount involved exceeded, or will exceed, $120,000 and in which any director, executive officer, holder of five percent or more of any class of our capital stock or any member of the immediate family of, or entities affiliated with, any of the foregoing persons, had, or will have, a direct or indirect material interest.interest, except as described in the next paragraph. Executive Officer and Director Compensation
Employment and Separation Agreements
We haveDr. Homcy resigned from our Board of Directors, effective June 18, 2019. Effective June 19, 2019, we entered into offer letters or employment agreementsa consulting agreement with each of Ted W. Love, M.D., Jeffrey Farrow, Tricia SuvariDr. Homcy, pursuant to which Dr. Homcy serves as a strategic consultant to the Company and certainas an observer on the Research and Development Committee of our executive officers,Board of Directors. As consideration for such consulting services, Dr. Homcy is entitled to receive a cash retainer of $2,500 per month and a termination letter agreement with Eleanor L. Ramos, M.D. For more information regarding these arrangements, see “Compensationat the start of Executive Officers—Employment Arrangements with Our Named Executive Officers.”
Equity Awards
For information regardingthe consultancy received (i) aone-time,up-front payment of $7,500 and (ii) an award of RSUs for 4,000 shares of the Company’s common stock, option awards and other equity incentive awardswhich were granted on June 19, 2019. The fair market value for such RSUs as of the date of grant was $234,200. The RSUs will vest on the first anniversary of the grant date, subject to our named executive officers and directors, see “Election of Directors—Director Compensation” and “Compensation of Executive Officers.”Dr. Homcy’s continued service under the consulting agreement.
Indemnification Agreements We have entered into agreements to indemnify our directors and executive officers. These agreements will, among other things, require us to indemnify these individuals for certain expenses (including attorneys’ fees), judgments, fines and settlement amounts reasonably incurred by such person in any action or proceeding, including any action by or in our right, on account of any services undertaken by such person on behalf of our company or that person’s status as a member of our board of directors to the maximum extent allowed under Delaware law. Procedures for Approval of Related Person Transactions The Audit Committee conducts an appropriate review of all related party transactions for potential conflict of interest situations on an ongoing basis, and the approval of the Audit Committee is required for all such transactions. The Audit Committee follows the policies and procedures set forth in our Related Person Transaction Policy in order to facilitate such review. The Related Person Transaction Policy is written. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS The following table sets forth the beneficial ownership information of our common stock by: each person known to us to be the beneficial owner of more than 5% of our common stock as of March 31, 2017;2020; each named executive officer;NEO; each of our directors;current director; and all of ourcurrent executive officers and directors as a group. We have based our calculation of the percentage of beneficial ownership of 43,602,80460,886,058 shares of common stock outstanding on March 31, 2017.2020. Each individual or entity shown in the table has furnished information with respect to beneficial ownership. The information with respect to each individual or entity is as of March 31, 2017,2020, unless otherwise noted. The information with respect to certain significant stockholders is based on filings by the beneficial owners with the SEC pursuant to sectionSections 13(d) and 13(g) of the Exchange Act. We have determined beneficial ownership in accordance with the SEC’s rules. These rules��rules generally attribute beneficial ownership of securities to persons who possess sole or shared voting power or investment power with respect to those securities. In addition, the rules include shares of common stock issuable pursuant to the exercise of stock options that are either immediately exercisable or exercisable on or before May 30, 2017,2020, which is 60 days after March 31, 2017.2020. These shares are deemed to be outstanding and beneficially owned by the person holding those stock options for the purpose of computing the percentage ownership of that person, but they are not treated as outstanding for the purpose of computing the percentage ownership of any other person. Unless otherwise indicated, the persons or entities identified in this table have sole voting and investment power with respect to all shares shown as beneficially owned by them, subject to applicable community property laws. | | | | | | | | | Beneficial Owner(1) | | Number of Shares
Beneficially Owned | | | Percentage
of Shares
Beneficially
Owned | | 5% or Greater Stockholders: | | | | | | | | | Entities affiliated with Fidelity(2)
245 Summer Street,
Boston, MA 02110 | | | 5,597,467 | | | | 12.8 | % | Entities affiliated with Third Rock Ventures(3)
29 Newbury Street, 3rd Floor,
Boston, Massachusetts 02116 | | | 4,760,904 | | | | 10.9 | % | Entities affiliated with Perceptive(4)
51 Astor Place, 10th Floor,
New York, NY 10003 | | | 3,128,978 | | | | 7.2 | % | Entities affiliated with BlackRock(5)
55 East 52nd Street,
New York, NY 10055 | | | 2,226,487 | | | | 5.1 | % |
| | | | | | | | | Beneficial Owner(1) | | Number of Shares
Beneficially Owned | | | Percentage of Shares
Beneficially Owned | | 5% or Greater Stockholders: | | | | | | | | | Entities affiliated with Fidelity(2) 245 Summer Street, Boston, MA 02210 | | | 6,651,295 | | | | 10.9 | % | Entities affiliated with Perceptive(3) 51 Astor Place, 10th Floor, New York, NY 10003 | | | 5,865,726 | | | | 9.6 | % | Entities affiliated with T. Rowe Price Associates, Inc.(4) 100 E. Pratt Street, Baltimore, MD 21202 | | | 5,656,185 | | | | 9.3 | % | Entities affiliated with The Vanguard Group(5) 100 Vanguard Blvd. Malvern, PA 19355 | | | 5,471,462 | | | | 9.0 | % | Entities affiliated with BlackRock(6) 55 East 52nd Street, New York, NY 10055 | | | 4,855,173 | | | | 8.0 | % | Entities affiliated with Bank of America Corporation(7) 100 N Tryon Street, Charlotte, NC 28255 | | | 4,698,143 | | | | 7.7 | % |
| | | | | | | | | Beneficial Owner(1) | | Number of Shares
Beneficially Owned | | | Percentage
of Shares
Beneficially
Owned | | Named Executive Officers and Directors: | | | | | | | | | Ted W. Love, M.D.(6) | | | 1,158,489 | | | | 2.7 | % | Jeffrey Farrow(7) | | | 32,312 | | | | * | | Tricia Suvari | | | — | | | | * | | Eleanor L. Ramos, M.D.(8) | | | 196,326 | | | | * | | Michael W. Bonney(9) | | | 26,250 | | | | * | | Willie L. Brown, Jr.(10) | | | 55,433 | | | | * | | Charles Homcy, M.D.(11) | | | 268,078 | | | | * | | Scott W. Morrison(12) | | | 27,083 | | | | * | | Deval L. Patrick(13) | | | 47,219 | | | | * | | Mark L. Perry(14) | | | 60,433 | | | | * | | Glenn F. Pierce, M.D., Ph.D.(15) | | | 26,250 | | | | * | | Philip A. Pizzo, M.D.(16) | | | 30,416 | | | | * | | All directors and executive officers as a group (14 persons)(17) | | | 2,315,196 | | | | 5.3 | % |
| | | | | | | | | Beneficial Owner(1) | | Number of Shares
Beneficially Owned | | | Percentage of Shares
Beneficially Owned | | Named Executive Officers and Directors: | | | | | | | | | Ted W. Love, M.D.(8) | | | 1,309,141 | | | | 2.1 | % | Jeffrey Farrow(9) | | | 215,071 | * | | | * | | Brian Cathers(10) | | | 21,647 | * | | | * | | David Johnson(11) | | | 55,591 | * | | | * | | Joshua Lehrer-Graiwer(12) | | | 3,220 | * | | | * | | Willie L. Brown, Jr.(13) | | | 106,903 | * | | | * | | Scott W. Morrison(14) | | | 81,875 | * | | | * | | Mark L. Perry(15) | | | 101,903 | * | | | * | | Glenn F. Pierce, M.D., Ph.D.(16) | | | 81,875 | * | | | * | | Philip A. Pizzo, M.D.(17) | | | 51,875 | * | | | * | | Dawn Svoronos(18) | | | 21,041 | * | | | * | | Wendy Yarno(19) | | | 46,041 | * | | | * | | All directors and executive officers as a group (16 persons)(20) | | | 2,615,993 | | | | 4.2 | % |
| * | Represents beneficial ownership of less than 1% of the shares of common stock. |
| (1) | Unless otherwise indicated, the address for each beneficial owner is c/o Global Blood Therapeutics, Inc., 400 East Jamie Court, Suite 101,181 Oyster Point Boulevard, South San Francisco, CA 94080. |
| (2) | Based solely on a report on Schedule 13G/A filed with the SEC on February 14, 2017,10, 2020, which indicates that FMR LLC had sole voting power with respect to 616,5051,549,966 shares of common stock and had sole dispositive power over 5,597,4676,651,295 shares of common stock. Abigail P. Johnson is a Director, the Vice Chairman, and the Chief Executive Officer and the President of FMR LLC. Members of the Johnson family including Abigail P. Johnson, are the predominant owners, directly or through trusts, of Series B voting common shares of FMR LLC, representing 49% of the voting power of FMR LLC. The Johnson family group and all other Series B shareholders have entered into a shareholders’ voting agreement under which all Series B voting common shares will be voted in accordance with the majority vote of Series B voting common shares. Accordingly, through their ownership of voting common shares and the execution of the shareholders’ voting agreement, members of the Johnson family may be deemed, under the Investment Company Act of 1940, to form a controlling group with respect to FMR LLC. Neither FMR LLC nor Abigail P. Johnson has the sole power to vote or direct the voting of the shares owned directly by the various investment companies registered under the Investment Company Act (“Fidelity Funds”) advised by Fidelity Management & Research Company (“FMR Co”), a wholly owned subsidiary of FMR LLC, which power resides with the Fidelity Funds’ Boards of Trustees. Fidelity Management & Research Company carries out the voting of the shares under written guidelines established by the Fidelity Funds’ Boards of Trustees. |
| (3) | Based solely on a report on Schedule 13G/A filed with the SEC on February 13, 2017, a report on Form 4 filed with the SEC on March 6, 201714, 2020, which indicates that Perceptive Advisors LLC and a report on Form 4 filed with the SEC on April 6, 2017, which indicate that (i) Third Rock Ventures II, L.P. (“TRV II”) directly owned, andJoseph Edelman had shared voting power and dispositive power over 4,475,1915,865,726 shares of common stock, all of which are held by Perceptive Life Sciences Master Fund Ltd (the “Fund”). Perceptive Advisors LLC serves as the investment manager to the Fund. Joseph Edelman is the managing member of Perceptive Advisors LLC. |
| (4) | Based solely on a report on Schedule 13G/A filed with the SEC on February 14, 2020, which indicates that T. Rowe Price Associates, Inc. had sole voting power with respect to 897,781 shares of common stock and had sole dispositive power over 5,656,185 shares of common stock. |
| (5) | Based solely on a report on Schedule 13G/A filed with the SEC on February 12, 2020, which indicates that The Vanguard Group had (i) sole voting power with respect to 122,827 shares of common stock, (ii) Third Rock Ventures III, L.P. (“TRV III”) directly owned, and had shared voting power andwith respect to 7,775 shares of common stock, (iii) sole dispositive power over 285,7135,348,702 shares of common stock. Each of Third Rock Ventures GP II, L.P. (“TRV GP II”), the sole general partner of TRV II,stock and TRV GP II, LLC (“TRV GP II LLC”), the sole general partner of TRV GP II, and Mark Levin, Kevin P. Starr and Robert I. Tepper, the managing members of TRV GP II LLC, may be deemed to have voting and investment(iv) shared dispositive power over the122,760 shares held of record by TRV II, and each of Third Rock Ventures GP III, LP (“TRV GP III”), the sole general partner of TRV III, and TRV GP III, LLC (“TRV GP III LLC”), the sole general partner of TRV GP III, and Mark Levin, Kevin P. Starr and Robert I. Tepper, the managing managers of TRV GP III LLC, may be deemed to have voting and investment power over the shares held of record by TRV III.common stock. |
(4)(6) | Based solely on a report on Schedule 13G/A filed with the SEC on February 5, 2020, which indicates that BlackRock, Inc. had sole voting power with respect to 4,708,151 shares of common stock and had sole dispositive power over 4,855,173 shares of common stock. |
| (7) | Based solely on a report on Schedule 13G filed with the SEC on February 14, 2017,2020, which indicates that Perceptive Advisors LLC and Joseph EdelmanBank of America Corporation had shared voting power and dispositive power over |
| 3,128,978 shares of common stock, 2,963,203 of which are held by Perceptive Life Sciences Master Fund Ltd (the “Fund”), and 165,775 of which are held in a trading account (the “Account”). Perceptive Advisors LLC serves as the investment manager to each of the Fund and the Account. Joseph Edelman is the managing member of Perceptive Advisors LLC. |
(5) | Based solely on a report on Schedule 13G filed with the SEC on January 30, 2017, which indicates that BlackRock, Inc. had sole voting power with respect to 2,200,1004,697,667 shares of common stock and had soleshared dispositive power over 2,226,4874,698,143 shares of common stock. |
(6)(8) | Includes (i) 1,144,4271,077,450 shares of common stock held by Dr. Love, 450,711 shares of which would be subject to our right of repurchase;Love; (ii) 5,000 shares of common stock held by Dr. Love’s two daughters, as to which Dr. Love disclaims beneficial ownershipownership; and (iii) 9,062226,691 shares of common stock that Dr. Love has the right to acquire from us within 60 days of March 31, 20172020 pursuant to the exercise of stock options. |
(7)(9) | Includes 32,312(i) 33,464 shares of common stock held by Mr. Farrow; and (ii) 181,607 shares of common stock that Mr. Farrow has the right to acquire from us within 60 days of March 31, 20172020 pursuant to the exercise of stock options. |
(8)(10) | Includes (i) 12,4705,179 shares of common stock held by Dr. Ramos,Mr. Cathers; and (ii) 1,000 shares of common stock held by Dr. Ramos’ step-daughter, and (iii) 182,856 shares of common stock that Dr. Ramos had the right to acquire from us within 60 days of October 24, 2016 pursuant to the exercise of stock options. Dr. Ramos’ employment with the Company ended on October 24, 2016, and her ownership information is presented as of such date. |
(9) | Includes 26,25016,468 shares of common stock that Mr. BonneyCathers has the right to acquire from us within 60 days of March 31, 20172020 pursuant to the exercise of stock options. |
(10)(11) | Includes (i) 21,42813,185 shares of common stock held by Mr. Brown, 9,373Johnson; and (ii) 42,406 shares of which would be subjectcommon stock that Mr. Johnson has the right to our rightacquire from us within 60 days of repurchase;March 31, 2020 pursuant to the exercise of stock options. |
| (12) | Includes (i) 2,595 shares of common stock held by Mr. Lehrer-Graiwer and (ii) 34,005625 shares of common stock that Mr. Lehrer-Graiwer has the right to acquire from us within 60 days of March 31, 2020 pursuant to the exercise of stock options. |
| (13) | Includes (i) 31,428 shares of common stock held by Mr. Brown; and (ii) 75,475 shares of common stock that Mr. Brown has the right to acquire from us within 60 days of March 31, 20172020 pursuant to the exercise of stock options. |
(11)(14) | Includes (i) 237,728 shares of common stock held by Dr. Homcy; (ii) 1,600 shares of common stock held by The Charles J. Homcy Irrevocable Trust UA 2/18/99; and (iii) 28,750 shares of common stock that Dr. Homcy has the right to acquire from us within 60 days of March 31, 2017 pursuant to the exercise of stock options. |
(12) | Includes 27,08381,875 shares of common stock that Mr. Morrison has the right to acquire from us within 60 days of March 31, 20172020 pursuant to the exercise of stock options. |
(13)(15) | Includes (i) 2,500 shares of common stock held by Mr. Patrick; and (ii) 44,719 shares of common stock that Mr. Patrick has the right to acquire from us within 60 days of March 31, 2017 pursuant to the exercise of stock options. |
(14) | Includes (i) 26,428 shares of common stock held by Mr. Perry, 10,712 shares of which would be subject to our right of repurchase;Perry; and (ii) 34,00575,475 shares of common stock that Mr. Perry has the right to acquire from us within 60 days of March 31, 20172020 pursuant to the exercise of stock options. |
(15)(16) | Includes 26,25081,875 shares of common stock that Dr. Pierce has the right to acquire from us within 60 days of March 31, 20172020 pursuant to the exercise of stock options. |
(16)(17) | Includes 30,41651,875 shares of common stock that Dr. Pizzo has the right to acquire from us within 60 days of March 31, 20172020 pursuant to the exercise of stock options. |
(17)(18) | Includes 21,041 shares of common stock that Ms. Svoronos has the right to acquire from us within 60 days of March 31, 2020 pursuant to the exercise of stock options. |
| (19) | Includes 46,041 shares of common stock that Ms. Yarno has the right to acquire from us within 60 days of March 31, 2020 pursuant to the exercise of stock options. |
| (20) | Includes the number of shares beneficially owned by the named executive officers and directors listed in the table above, as well asas: (i) 144,8553,527 shares of common stock held by Jung Choi, 71,426Lesley Calhoun and 36,223 shares of which would be subjectcommon stock that Ms. Calhoun has the right to our rightacquire from us within 60 days of repurchase,March 31, 2020 pursuant to the exercise of stock options; (ii) 161,508 shares of common stock held by Ms. Choi, 25,000 shares of common stock held in The 2005 William Park and Jung Choi Family Trust and 60,205154,719 shares of common stock that Ms. Choi has the right to acquire from us within 60 days of March 31, 20172020 pursuant to the exercise of stock options; (ii) 3,222 shares of common stock held by Peter Radovich, 12,500 shares of common stock held in The Radovich Family Trust and 88,946(iii) 2,406 shares of common stock that Mr. RadovichFink has the right to acquire from us within 60 days of March 31, 20172020 pursuant to the exercise of stock options; and (iii) 58,204(iv) 11,521 shares of common stock held by Hing L. Sham, Ph.D., 4,640 shares of which would be subject to our right of repurchaseMs. Suvari and 62,474124,906 shares of common stock that Dr. ShamMs. Suvari has the right to acquire from us within 60 days of March 31, 20172020 pursuant to the exercise of stock options. |
SECTION 16(a) BENEFICIAL OWNERSHIP REPORTING COMPLIANCE
Section 16(a) of the Exchange Act requires our officers and directors, and persons who own more than 10% of a registered class of our equity securities, to file reports of ownership and changes in ownership (Forms 3, 4 and 5) with the SEC. Officers, directors and greater than 10% stockholders are required to furnish us with copies of all such forms which they file.
To our knowledge, based solely on our review of such reports or written representations from certain reporting persons, we believe that all of the filing requirements applicable to our officers, directors, greater than 10% beneficial owners and other persons subject to Section 16 of the Exchange Act were complied with during the year ended December 31, 2016, except that one Form 4 filed on behalf of Dr. Love, to report the repurchase of unvested restricted stock that was forfeited, was filed late due to administrative error.
The following Audit Committee Report is not considered proxy solicitation material and is not deemed filed with the Securities and Exchange Commission. Notwithstanding anything to the contrary set forth in any of the Company’s filings made under the Securities Act of 1933 or the Exchange Act that might incorporate filings made by the Company under those statutes, the Audit Committee Report shall not be incorporated by reference into any prior filings or into any future filings made by the Company under those statutes.
AUDIT COMMITTEE REPORT
The Audit Committee of the Board of Directors (the “Audit Committee”) has furnished this report concerning the independent audit of the Company’s financial statements. Each member of the Audit Committee meets the enhanced independence standards established by the Sarbanes-Oxley Act of 2002 and rulemaking of the Securities and Exchange Commission (the “SEC”) and the NASDAQ Stock Market regulations. A copy of the Audit Committee Charter is available on the Company’s website at http://www.globalbloodtx.com.
The Audit Committee’s responsibilities include assisting the Board of Directors regarding the oversight of the integrity of the Company’s financial statements, the Company’s compliance with legal and regulatory requirements, the independent registered public accounting firm’s qualifications and independence, and the performance of the independent registered public accounting firm.
In fulfilling its oversight responsibilities, the Audit Committee reviewed and discussed the Company’s financial statements for the fiscal year ended December 31, 2016 with the Company’s management and KPMG LLP. In addition, the Audit Committee has discussed with KPMG LLP, with and without management present, their evaluation of the Company’s internal controls over financial reporting and overall quality of the Company’s financial reporting. The Audit Committee also discussed with KPMG LLP the matters required to be discussed by Statement on Auditing Standards No. 114 (formerly SAS 61), as amended (AICPA,ProfessionalStandards, Vol. 1 AU section 380), as adopted by the Public Company Accounting Oversight Board in Rule 3200T. The Audit Committee also received the written disclosures and the letter from KPMG LLP required by the Public Company Accounting Oversight Board Rule 3526 and the Audit Committee discussed the independence of KPMG LLP with that firm.
Based on the Audit Committee’s review and discussions noted above, the Audit Committee recommended to the Board of Directors, and the Board of Directors approved, that the audited financial statements be included in the Company’s Annual Report for the fiscal year ended December 31, 2016.
The Audit Committee and the Board of Directors have recommended the selection of KPMG LLP as the Company’s independent registered public accounting firm for the year ending December 31, 2017.
| AUDIT COMMITTEE | | SCOTT W. MORRISON, CHAIRMAN | DEVAL L. PATRICK | MARK L. PERRY |
HOUSEHOLDING OF PROXY MATERIALS We have made available a procedure approved by the SEC known as “householding.” This procedure allows multiple stockholders residing at the same address the convenience of receiving a single copy of our Notice, Annual Report onForm 10-K and proxy materials, as applicable. This allows us to save money by reducing the number of documents we must print and mail, and helps protect the environment as well. Householding is available to both registered stockholders (i.e., those stockholders with certificates registered in their name) and streetname holders (i.e., those stockholders who hold their shares through a brokerage). Registered Stockholders If you are a registered stockholder and would like to consent to a mailing of proxy materials and other stockholder information only to one account in your household, as identified by you, we will deliver or mail a single copy of our Annual Report and proxy materials for all registered stockholders residing at the same address. Your consent will be perpetual unless you revoke it, which you may do at any time by contacting the HouseholdingProxy Department of AmericanContinental Stock Transfer & Trust Company, LLC, (“AST”),or Continental, at One Embarcadero Center, Suite 530, San Francisco, CA 94111.1 State Street, 30th Floor, New York, NY 10004. Registered stockholders who have not consented to householding will continue to receive copies of Annual Reports and proxy materials for each registered stockholder residing at the same address. As a registered stockholder, you may elect to participate in householding and receive only a single copy of Annual Reports or proxy statements for all registered stockholders residing at the same address by contacting ASTContinental as outlined above. Street Name Holders Stockholders who hold their shares through a brokerage may elect to participate in householding or revoke their consent to participate in householding by contacting their respective brokers. OTHER MATTERS We are not aware of any matters that may come before the meeting other than those referred to in the Notice. If any other matter shall properly come before the Annual Meeting, however, the persons named in the accompanying proxy intend to vote all proxies in accordance with their best judgment. Accompanying this Proxy Statement is our Annual Report. Copies of our Annual Report are available free of charge on our website at www.globalbloodtx.comhttps://ir.gbt.com/sec-filings or you can request a copy free of charge by calling Investor Relations at(415) 946-1090 650.741.7730 or sending ane-mail request to investor@globalbloodtx.com.investor@gbt.com. Please include your contact information with the request. | | By Order of the Board of Directors, | | Global Blood Therapeutics, Inc. | | /s/ Ted W. Love, M.D.
| | Ted W. Love, M.D. | | President and Chief Executive Officer |
April 28, 20172020 | | | 
GLOBAL BLOOD THERAPEUTICS, INC. 400 EAST JAMIE COURT, SUITE 101181 OYSTER POINT BOULEVARD
SOUTH SAN FRANCISCO, CA 94080 | | VOTE BY INTERNET Before The Meeting -Go towww.proxyvote.com Use the Internet to transmit your voting instructions and for electronic delivery of information up until 11:59 P.M.p.m. Eastern Time the day before the meeting date.on June 16, 2020. Have your proxy card in hand when you access the web site and follow the instructions to obtain your records and to create an electronic voting instruction form. | | ELECTRONIC DELIVERY OF FUTURE PROXY MATERIALSDuring The Meeting -Go towww.virtualshareholdermeeting.com/GBT2020
If you would like to reduce
You may attend the costs incurred by our company in mailing proxy materials, you can consent to receiving all future proxy statements, proxy cards and annual reports electronicallymeeting via e-mail or the Internet. To sign up for electronic delivery, please follow the instructions above to vote using the Internet and when prompted, indicatevote during the meeting. Have the information that you agree to receive or access proxy materials electronicallyis printed in future years.the box marked by the arrow available and follow the instructions. | | VOTE BY PHONE - 1-800-690-6903 Use any touch-tone telephone to transmit your voting instructions up until 11:59 P.M.p.m. Eastern Time the day before the meeting date.on June 16, 2020. Have your proxy card in hand when you call and then follow the instructions. | | | | | VOTE BY MAIL Mark, sign and date your proxy card and return it in the postage-paid envelope we have provided or return it to Vote Processing, c/o Broadridge, 51 Mercedes Way, Edgewood, NY 11717. |
| | | | | TO VOTE, MARK BLOCKS BELOW IN BLUE OR BLACK INK AS FOLLOWS: E28324-P90083
| | D15136-P35794 KEEP THIS PORTION FOR YOUR RECORDS — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — —
|
— — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — | | | DETACH AND RETURN THIS PORTION ONLY | THIS PROXY CARD IS VALID ONLY WHEN SIGNED AND DATED. |
|



